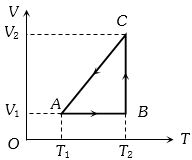
$1\, mol$ વાયુની ચક્રીય પ્રક્રિયા આપેલી છે.તો $AB,BC$ અને $CA$ માં થતું કાર્ય

Medium
c
(c) Process \(AB\) is isochoric, \(\therefore\) \({W_{AB}} = P\,\Delta V = 0\)
(c) Process \(AB\) is isochoric, \(\therefore\) \({W_{AB}} = P\,\Delta V = 0\)
Process \(BC\) is isothermal \(\therefore\) \({W_{BC}} = R{T_2}.\ln \left( {\frac{{{V_2}}}{{{V_1}}}} \right)\)
Process \(CA\) is isobaric
\(\therefore {W_{CA}} = - \,P\Delta V\)\( = - \,R\Delta T\)\( = - \,R({T_1} - {T_2})\)\( = R({T_2} - {T_1})\)
(Negative sign is taken because of compression)
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1View Solutionવ્યવહારમાં બધા જ હીટ એન્જિનો કાર્નોટ એન્જિન કરતાં ઓછી કાર્યક્ષમતા ધરાવે છે, કારણ કે...
- 2કાર્નોટ એન્જિનમાં ઠારણવ્યવસ્થાનું તાપમાન $27 °C$ અને ઉષ્માપ્રાપ્તિસ્થાનનું તાપમાન $927 °C$ છે. જો એન્જિન દ્વારા ઉષ્માપ્રાપ્તિસ્થાનમાંથી ઠારણવ્યવસ્થામાં ઉષ્મા ઠાલવવા માટે થતું કાર્ય $12.6 × 10^{6} J$ હોય, તો ઉષ્માપ્રાપ્તિસ્થાનમાંથી શોષેલી ઉષ્મા કેટલી થાય ?View Solution
- 3એક એક પરમાણ્વિક વાયુનું દબાણ $P$, કદ $V$ અને તાપમાન $T$ ને સમતાપી રીતે વિસ્તરણ કરવામાં આવે તો તેનું કદ $2V$ અને અંતિમ દબાણ $P_i$ થાય.જો તે જ વાયુને સમોષ્મી રીતે વિસ્તરણ કરવામાં આવે તો તેનું કદ $2V$ અને અંતિમ દબાણ $P_a$ થાય તો ગુણોત્તર $\frac{{{P_a}}}{{{P_i}}}$ કેટલો થાય?View Solution
- 4એક મોલ $O _2$ વાયુનું કદ એ $0 ^{\circ} C$ એ રહેલા $22.4 \;ltr$ જેટલુ છે. તેને સમતાપી રીતે $1\; atm$ દબાણમાં દબાવવામાં આવે છે જેથી તેનું કદ $11.2 \;ltr$ થાય. આ પ્રક્રિયામાં થતું કાર્ય ......$J$ હશે?View Solution
- 5તંત્ર વડે થતું કાર્ય $333 \,cal $ હોય,તો તંત્રની આંતરિક ઊર્જામાં થતો ફેરફાર $167 \,cal$ હોય,તો તંત્રને ....... $cal$ ઉષ્મા આપવી પડે?View Solution
- 6રેફરીઝરેટર ઉષ્માપ્રાપ્તિસ્થાન માંથી $800\, J$ લે છે, અને ઠારણ વ્યવસ્થામાં $500\, J$ ઉષ્મા ગુમાવે છે તો તેનો પરફોર્મન્સ ગુણાંક કેટલો હશે?View Solution
- 7બે વાયુના સમોષ્મી પ્રક્રિયાના ગ્રાફ આપેલા છે.તો ગ્રાફ $1$ અને $2$ કયાં વાયુના હશે.View Solution
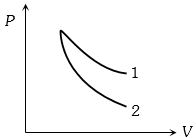
- 8View Solutionથરર્મોડાઇનેમિકસનો પ્રથમ નિયમ નીચે પૈકી કોની વિશેષ સ્થિતિ દર્શાવે છે?
- 9રેફ્રિજરેટરમાં કાર્નોટ એન્જિન $250\, K$ અને $300\, K$ વચ્ચે કાર્ય કરે છે.તે નીચા તાપમાનના સ્ત્રોતમાથી $500\, cal$ ઉષ્મા મેળવે છે.તો રેફ્રિજરેટરમાં થતું કાર્ય ..... $J$ હશે.View Solution
- 10$5.6$ લીટર હિલિયમને $ STP$ એ સમોષ્મી રીતે $0.7$ લીટર સુધી સંકોચવામાં આવે છે. પ્રારંભીક તાપમાન $T_1$ લેતા પ્રક્રિયા દરમિયાન થતુ કાર્ય...?View Solution
