પાણીના ઠારણબિંદુ અને ઉત્કલનબિંદુ વચ્ચે કાર્ય કરતાં એક આદર્શ ઉષ્માયંત્રની કાર્યક્ષમતા ($\%$ માં) કેટલી થાય?
NEET 2018,JEE MAIN 2022, Medium
a
Efficiency of an ideal heat engine,
Efficiency of an ideal heat engine,
\(\eta = \left( {1 - \frac{{{T_2}}}{{{T_1}}}} \right)\)
Freezing point of water \( = {0^ \circ }C = 273\,K\)
Boiling point of water\( = {100^ \circ }C = \left( {100 + 273} \right)K\)
\( = 373\,K\)
\(T_2\) Sink temperature\(=273 K\)
\(T_1\) Source temperature \(=373 K\)
\(\% \eta = \left( {1 - \frac{{{T_2}}}{{{T_1}}}} \right) \times 100 = \left( {1 - \frac{{273}}{{373}}} \right) \times 100\)
\( = \left( {\frac{{100}}{{373}}} \right) \times 100 = 26.8\% \)
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1કાર્નોટ એન્જિન ઉષ્માનું છઠા ભાગનું કાર્યમાં રૂપાંતર કરે છે. જયારે ઠારણ વ્યવસ્થાનું તાપમાન $62^oC$ ઘટાડવામાં આવે, ત્યારે કાર્યક્ષમતા બમણી થાય છે. તો ઉષ્મા પ્રાપ્તિસ્થાન અને ઠારણ વ્યવસ્થાનું તાપમાન કેટલું હશે?View Solution
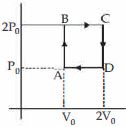
- 2આકૃતિમાં દર્શાવ્યા અનુસાર, હિલિયમ વાયુ $ABCDA$ ચક્રમાંથી પસાર થાય છે. (જે બે સમકદ અને બે સમદાબી રેખાઓ ધરાવે છે.) આ ચક્રની કાર્યક્ષમતા આશરે ....... $\%$ થાય. (વાયુને આદર્શ વાયુ જેવો ધારો)View Solution
- 3$1/3$ પરફોમન્સ ગુણાંક ધરાવતા રેફ્રીજરેટર $200 J$ ઉષ્મા મુક્ત કરે છે તો કાર્યકારી પદાર્થ પર થતુ કાર્ય........... $J$ ?View Solution
- 4$A \rightarrow B \rightarrow C$ જવા માટે તંત્ર પર થતું કાર્ય $50 J$ અને તંત્રને અપાતી ઊર્જા $20cal$ છે.તો $A$ અને $C$ વચ્ચે આંતરિક ઊર્જામાં થતો ફેરફાર ...... $J$View Solution
- 5${27^o}C$ તાપમાને રહેલ હિલિયમનું કદ $8$ લિટર છે.તેનું અચાનક સંકોચન કરીને કદ $1$ લિટર કરતાં વાયુનું તાપમાન ....... $^oC$ થાય? $[\gamma = 5/3]$View Solution
- 6એક મોલ વાયુને શરૂઆતની સ્થિતિ $(P_1, V_1,T)$ થી અંતિમ સ્થિતિ $(P_2, V_2,T)$ સમતાપી પ્રક્રિયા દ્વારા લઈ જવામાં આવે તો પ્રક્રિયા દરમિયાન એન્ટ્રોપીમાં થતો ફેરફાર કેટલો હશે?View Solution
- 7View Solutionનીચેનામાંથી થરમૉડાયનેમિક્સનો ક્યો નિયમ આંતરિક ઉર્જા પદ ને વ્યાખ્યાયિત કરે છે ?
- 8એ આભાસી વાયુ સમોષ્મી રીતે વિસ્તરણ પામે છે કે જેથી તેનું કદ $8$ લીટર થી વધીને $27$ લીટર થાય છે.જો વાયુના અંતિમ દબાણ અને પ્રારંભિક દબાણનો ગુણોતર $\frac{16}{81}$ હોય, તો $\frac{C_p}{C_v}$ ગુણોતર $.......$View Solution
- 9$NTP$ એ રહેલા વાયુનું સંકોચન કરી કદ ચોથા ભાગનું કરવામાં આવે છે.જો $ \gamma $ = $ \frac{3}{2} $ હોય,તો અંતિમ દબાણ ....... વાતાવરણ થશે?View Solution
- 10કાર્નોટ એન્જિનની કાર્યક્ષમતા $50\%$ હોય,જયારે ઠારણ વ્યવસ્થાનું તાપમાન $7 °C$ હોય છે.કાર્યક્ષમતા $70\%$ કરવા માટે ઉષ્મા પ્રાપ્તિસ્થાનનું તાપમાન ...... $K$ વધારવું પડે?View Solution
