$0^{\circ}\,C$ અને $760\,mm\,Hg$ પર માપેલ $H _2$ વાયુના $8.40\,mL$ નો $17\,mg$ હાઈડ્રોકાર્બન (M.F. $C _{10} H _{16}$ ) ઉપયોગ કરે છે. આજ હાઈડ્રોકાર્બનના ઓઝોનાલિસીસથી મળતી નીપજો (આકૃતિ) હાઈડ્રોકાર્બન માં હાજર દ્વિબંધ (ધો)ની સંખ્યા $..........$ છે.
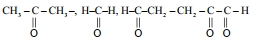
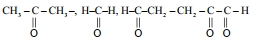
JEE MAIN 2023, Diffcult
a
Moles of hydrocarbon $=\frac{17 \times 10^{-3}}{136}=1.25 \times 10^{-4}$
Moles of hydrocarbon $=\frac{17 \times 10^{-3}}{136}=1.25 \times 10^{-4}$
Mole of $H _2$ gas
$\Rightarrow 1 \times \frac{8.40}{1000}= n \times 0.0821 \times 273$
$\Rightarrow n =3.75 \times 10^{-4}$
Hydrogen molecule used for 1 molecule of hydrocarbon is 3
$=\frac{3.75 \times 10^{-4}}{1.25 \times 10^{-4}}=3$
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1View Solutionસૂર્યપ્રકાશ હેઠળ આલ્કેન્સના હેલોજનીકરણમાં સૌથી વધુ ક્રિયાશીલ હેલોજન કયો છે?
- 2View Solutionઇલેકટ્રોનની વિસ્થાપનના નીચેનામાંથી કઇ પ્રક્રિયા બેન્ઝિન કરતાં ધીમી છે ?
- 3નીચેની પ્રક્રિયાની નિપજો કઈ છે ?View Solution
$C{H_3}C \equiv \,C\,C{H_2}C{H_3}\mathop {\xrightarrow[{(2)\;Hydrolysis}]{}}\limits^{(1)\;\;{O_3}} $ .......
- 4View Solutionસૌથી સ્થાયી કાર્બોનિયમ આયન
- 5View Solutionનીચેના પૈકી ક્યુ સંયોજન ઇલેક્ટ્રોન અનુરાગી બ્રોમિનેશનમાં બેન્ઝિન કરતા ધીમી પ્રક્યિા કરે છે ?
- 6$2-$ હેક્ઝીનને ટ્રાન્સ$-2-$હેક્ઝેનમાં રૂપાંતરણ કરવા માટે નીચેનામાંથી કયું ઉપયોગ થાય છે?View Solution
- 7પેન્ટ$-2-$ઇન$-4-$આઇન માં સિગ્મા ( $\sigma$ ) અને પાઇ ( $\pi$ ) બંધની, સંખ્યા જણાવો.View Solution
- 8View Solutionનાઇટ્રોબેન્ઝિન ના નાઇટ્રેશનથી ..... બને છે.
- 9$2, 2, 3-$ ટ્રાયમિથાઇલ બ્યુટેનનો ઓક્ટેન આંક ............માનવામાં આવે છે.View Solution
- 10લિથિયમ ડાય $-૩-$ પેન્ટાઇલ ક્યુપ્રેટની ઇથાઇલ બ્રોમાઇડ સાથેની પ્રક્રિયાથી $C_7H_{16}$ આણ્વિય સૂત્ર ધરાવતો આલ્કેન મળે તો આલ્કેનનું બંધારણ ............ થશે.View Solution
