\(i)\) \(C + O _2 \longrightarrow O _2\)
\(\left.\begin{array}{cc}0.8 g & x \text { cal } \\ 12 g & (?)\end{array}\right\}=\frac{12 x }{0.8}=15 x\)
\(ii)\) \(C +\frac{1}{2} O _2 \rightarrow CO\)
\(\left.\begin{array}{cc}0.8 g & \text { y cal } \\ \therefore 12 g & \text { (?) }\end{array}\right\}=\frac{12 y }{0.8}=15 y\)
\((i) - (ii):\) \(CO +\frac{1}{2} O _2 \longrightarrow CO _2\)
\(\left.\begin{array}{r}28 \,g \rightarrow 15 x -15 y \\ \therefore 1.86\, g \rightarrow(?)\end{array}\right\}=\frac{1.86 \times 15( x - y )}{28}\)
\(=\frac{27.9(x-y)}{28}=x-y\)
Download our appand get started for free
Similar Questions
- 1જો $\mathrm{Br}_{2(l)}$ ની પરમાણ્વીયકરણ એન્થાલ્પી $\mathrm{x}\; \mathrm{kJ} / \mathrm{mol}$ અને $\mathrm{Br}_{2}$ માટે બંધ એન્થાલ્પી $y \;\mathrm{kJ} / \mathrm{mol}$ હોય તો તેઓ વચ્ચેનો સંબંધ જણાવો.View Solution
- 2$H_2$$_{(g)}$ $+ \frac{1}{2}O_2$$ _{(g)}$ $\rightarrow$$H_2$$O$$_{(l)}$; $\Delta$ $H_{298}$ $K$ $=$ $- 68.32\, Kcal$. તો $25\,^oC$ એ $1$ વાતાવરણ દબાણે પાણીના બાષ્પાયન ઉષ્મા $10.52 \,Kcal$ છે. $25\,^oC$ એ $1\, mole$ પાણીના બાષ્પની પ્રમાણિત નિર્માણ ઉષ્મા ( $kcal$ માં)શોધો.View Solution
- 3View Solutionવાયુના સ્વયંભૂ શોષણ માટે નીચેનામાંથી કયું વિધાન સાચું છે?
- 4એક એન્જિન $T_1$ તાપમાને $Q_1$ ઉષ્મા અને $T_2$ તાપમાને $Q_2$ ઉષ્મા ગ્રહણ કરે, ત્યારે એન્જિન દ્રારા થતું કાર્ય [$Q_1$ + $Q_2$] છે. અહી કયો વિકલ્પ યોગ્ય છે ?View Solution
- 5પ્રતિવર્તીં પ્રક્રિયા માટે $T = 300\,K,$ કદ વધીને $V_f = 1\,L$ થી$ V_f = 10\,L$ થાય છે. તો જો પ્રક્રિયા સમઉષ્મીય હોય તો $\Delta H$ ................. $\mathrm{kJ}$ શોધો.View Solution
- 6$0\,^oC$ તાપમાન ધરાવતા $54\, g$ બરફનુ $27\,^oC$ તાપમાન ધરાવતા પાણીમાં રૂપાંતર કરવા કેટલા ......$kJ$ ઊર્જાની જરૂર પડે ? $\left( {\Delta {H_{fusion}} = 6.01\,kJ\,mo{l^{ - 1}},{C_{p\left( {liquid} \right)}} = 4.18\,J\,{K^{ - 1}}\,{g^{ - 1}}} \right)$View Solution
- 7$NH_4OH$ ની $HCl$ સાથેની તટસ્થીકરણ એન્થાલ્પી $-51 .46\,kJ\,mol^{-1}$ અને $NaOH$ ની $HCl$ સાથેની તટસ્થીકરણ એન્થાલ્પી $-55. 90\,kJ\,mol^{-1}.$ છે. તો $NH_4OH$ ની આયનીકરણ ઊર્જા ......$kJ\,mol^{-1}$View Solution
- 8નીચે દર્શાવેલ પ્રક્રિયાને આધારે $ICl_{(g)}$ ની પ્રમાણિત સર્જનએન્થાલ્પી ગણો. આયોડિન અને ક્લોરિનની પ્રમાણિત સ્થિતિ અનુક્રમે $I_2$ $_{(s)}$ અને $Cl_2$$_{(s)}$ છે.View Solution
$(i)$ $Cl_2$$_{(g)}$ $=$ $2Cl_{(g)}$ , $\Delta H = 242.3$ કિલો જૂલ મોલ$^{-1}$
$(ii)$ $I_2$$_{(g)}$ $=$ $2I_{(g)}$, $\Delta H = 151.0$ કિલો જૂલ મોલ$^{-1}$
$(iii)$ $ICl_{(g)}$ $=$ $I_{(g)} +$ $Cl_{(g)}$, $\Delta H = 211.3$ કિલો જૂલ મોલ$^{-1}$
$(iv)$ $I_{2(s)}$ $=$ $I_{2(g)}$, $\Delta H = 62.76$ કિલો જૂલ મોલ$^{-1}$
.....કિલો જૂલ મોલ$^{-1}$
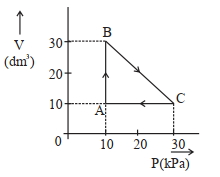
- 9$Image$ એક આદર્શ વાયુ બિંદુ $A$ થી શરૂઆત કરીને ચક્રિય સ્થાનાંતર કરે છે અને ઉપરની આકૃતિમાં દર્શાવ્યા પ્રમાણે દોરેલા પથ $\mathrm{A} \rightarrow \mathrm{B} \rightarrow \mathrm{C} \rightarrow \mathrm{A}$ દૃવારા તે જ બિંદુ પર પાછો ફરે છે. આ પ્રક્રમમાં થયેલ કુલ કાર્ય ____________$\mathrm{J}$ છે.View Solution
- 10નીચેના પૈકી ક્યા પ્રકમ સાથે એન્ટ્રોપીનો વધારો સંકળાયેલ છે ?View Solution
$(I)$ ધનનુ ગલન $(II)$ વાયુઓને મિશ્ર ક્રરવા
$(III)$ વાયુનુ સંકોચન $(IV)$ વાયુનુ વિસ્તરણ
