(a) $C{H_3} - C{H_2} - C{H_2} - C{H_3} + B{r_2}\xrightarrow[{{{130}\,^o}C}]{{Light}}$
$\mathop {C{H_3} - \mathop {CH}\limits_{\mathop {|\,\,\,\,\,}\limits_{Br\,\,} } - C{H_2} - C{H_3}}\limits_{\scriptstyle{\rm{2}} - {\rm{Bromo}}\,{\rm{butane}}\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,{\rm{ }}\atop \scriptstyle\,\,\,{\rm{(Main}}\,{\rm{product)}}} + \mathop {C{H_3} - C{H_2} - C{H_2} - C{H_2} - Br}\limits_{\scriptstyle{\rm{1}} - {\rm{Bromo}}\,{\rm{butane}}\atop \scriptstyle\,\,\,\,\,\,\,\,\,\,\,{\rm{(Minor)}}} $
$2-$ Bromobutane is the main product because
${2^o}$ carbonium ion is more stable than ${1^o}$.
Download our appand get started for free
Similar Questions
- 1View Solutionનીચેનામાંથી સૌથી વધારે બેઝિક સંયોજન છે...
- 2સલ્ફ્યુરિક એસિડ અને મર્ક્યુરી$ (II)$ સલ્ફેટની હાજરીમાં આલ્કાઇન હાઇડ્રેશન દ્વારા કીટોનની બનાવટ માં નીચેનામાંથીકયું મધ્યવર્તી છે?View Solution
- 3View Solutionજંતુનાશક ગેમેક્સિનનું રાસાયણિક નામ..... છે.
- 4ઇથાઇલ આયોડાઇડ પર આલ્કોહોલિક $KOH$ ની ક્રિયા દ્વારા રચાયેલ વાયુ જે આલ્કલાઇન $KMn{O_4}$ના ક્ષારીય દ્રાવણને રંગવિહીન કરે છે,તો તે વાયુ કયો હ?શેView Solution
- 5View Solution.......... નો ફ્રિડલ ક્રાફટ પ્રક્રિયામાં ઉપયોગ થતો નથી.
- 6નીચે બે વિધાનો આપેલા છે -View Solution
વિધાન $I$ : બેન્ઝિનના નાઈટ્રિશનમાં નીચનો તબક્કો સંકળાયેલ છે.
(Image)
વિધાન $II$ : લુઈસ બેઈઝ નો ઉપયોગ બેન્ઝિન ની ઈલેક્ટ્રોન અનુરાગી વિસ્થાપન માં પ્રોત્સાહિત (અભિવૃધ્ધિ) કરે છે.
ઉપરના વિધાનોના સંદર્ભમાં, નીચે આપેલા વિકલ્પોમાંથી સૌથી બંધબેસતો જવાબ પસંદ કરો.
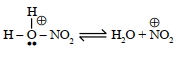
- 7View Solutionનીચેનામાંથી કોનો ઉપયોગ ઇથેનથી ઇથેનને અલગ કરવા માટે થતો નથી?
- 8View Solutionસિલ્વર પાઉડરની ક્લોરોફોર્મ સાથેની પ્રક્રિયાથી .... મળે છે.
- 9$125^{\circ} {C}$ પર પ્રકાશની હાજરીમાં વધુ પ્રમાણમાં આઇસોબ્યુટેન ${Br}_{2}$ સાથે પ્રક્રિયા પર નીચેનામાંથી કયું મુખ્ય નીપજ તરીકે આપે છે?View Solution
- 10ઇથાઇલ આયોડાઇડ પર આલ્કોહોલિક $KOH$ ની ક્રિયા દ્વારા રચાયેલ વાયુ જે આલ્કલાઇન $KMn{O_4}$ના ક્ષારીય દ્રાવણને રંગવિહીન કરે છે,તો તે વાયુ કયો હ?શેView Solution
