(a) \(C{H_3} - C{H_2} - C{H_2} - C{H_3} + B{r_2}\xrightarrow[{{{130}\,^o}C}]{{Light}}\)
\(\mathop {C{H_3} - \mathop {CH}\limits_{\mathop {|\,\,\,\,\,}\limits_{Br\,\,} } - C{H_2} - C{H_3}}\limits_{\scriptstyle{\rm{2}} - {\rm{Bromo}}\,{\rm{butane}}\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,{\rm{ }}\atop \scriptstyle\,\,\,{\rm{(Main}}\,{\rm{product)}}} + \mathop {C{H_3} - C{H_2} - C{H_2} - C{H_2} - Br}\limits_{\scriptstyle{\rm{1}} - {\rm{Bromo}}\,{\rm{butane}}\atop \scriptstyle\,\,\,\,\,\,\,\,\,\,\,{\rm{(Minor)}}} \)
\(2-\) Bromobutane is the main product because
\({2^o}\) carbonium ion is more stable than \({1^o}\).
Download our appand get started for free
Similar Questions
- 1View Solutionબેન્ઝિનમાં પ્રત્યેક કાર્બન-કાર્બન બંધનો બંધ-ક્રમાંક શું છે?
- 2View Solutionનીચેના પૈકી કોની હેલોજન એસિડ સાથેની પ્રક્રિયા સૌથી ધીમા દરથી થશે ?
- 3View Solutionટોલ્યુઇન અને ક્રોમાઇલ ક્લોરાઇડ પ્રક્રિયા કરી..... બનાવે છે.
- 4ઉપરોક્ત પ્રક્રિયામાં ગ્લાયોક્સેલનું પાયરુંઆલ્ડિહાઈડ નું પ્રમાણ શું છે? ?View Solution
(figure) $\xrightarrow[{(2)\,Zn}]{{(1)\,{O_3}}}\mathop {\begin{array}{*{20}{c}}
{\,\,\,\,O\,\,\,\,\,\,\,O\,\,} \\
{\,||\,\,\,\,\,\,\,\,\,||} \\
{H - C - C - H}
\end{array}}\limits_{Glyoxal} + $ $\mathop {\begin{array}{*{20}{c}}
{\,\,\,O\,\,\,\,\,O\,\,} \\
{\,||\,\,\,\,\,\,\,\,||} \\
{C{H_3} - C - C - C{H_3}}
\end{array}}\limits_{2,3 - Bu\tan edione} + \mathop {\begin{array}{*{20}{c}}
{\,\,\,\,\,\,O\,\,\,\,\,O} \\
{\,\,\,\,\,\,||\,\,\,\,\,\,||} \\
{C{H_3} - C - C - H}
\end{array}}\limits_{Pyrualdehyde} $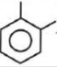
- 5$C_6H_{12}$ સંયોજન...... છે.View Solution
- 6View Solutionકયું ન્યૂનત્તમ ઉત્કલનબિદુ ધરાવે છે ?
- 7View Solutionપ્રકિયા ની મુખ્ય નીપજ કઈ છે ?
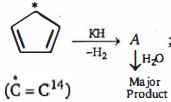
- 8View Solutionઆલ્કિનના આડકતરી રીતે જલીયકરણ કરવાથી માત્ર એક જ આલ્કોહોલ બનાવી શકાતો નથી. આ આલ્કોહોલ ...... છે.
- 9સાયક્લોપેન્ટિનની આલ્કલાઇન $KMnO_4$ સાથેની પ્રક્યિાથી ................ મળે છે.View Solution
- 10View Solutionનીચે પૈકી કોણ મહત્તમ સ્થાયીતા ધરાવે છે?
