The order of substituion in different alkanes is
\(3^o > 2^o > 1^o\)
Thus the bromination of \(2-\) methyl butane mainly gives \(2-\) Bromo \(-2-\) methyl butane
\(\mathop {\begin{array}{*{20}{c}}
{\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,C{H_3}} \\
{\,\,\,\,\,\,\,\,\,\,\,\,\,\,|\,\,\,\,} \\
{C{H_3} - C{H_2} - CH - C{H_3}}
\end{array}}\limits_{2 - methyl\,bu\tan e} \xrightarrow{{B{r_2}}}\)
\(\mathop {\mathop {\begin{array}{*{20}{c}}
{\,\,\,\,\,\,\,\,\,\,C{H_3}} \\
{\,\,\,\,\,|} \\
{C{H_3} - C{H_2} - CH - C{H_2}Br}
\end{array}}\limits_{1 - Bromo - 2\,methyl\,bu\tan e} }\limits_{(\min or)} \) \(+\) \(\underset{\begin{smallmatrix}
2-Bromo-2-methyl\,bu\tan e \\
\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,(major)
\end{smallmatrix}}{\mathop{\begin{matrix}
\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,C{{H}_{3}} \\
\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,| \\
C{{H}_{3}}-C{{H}_{2}}-C-C{{H}_{3}} \\
\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,| \\
\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,Br \\
\end{matrix}}}\,\)
Download our appand get started for free
Similar Questions
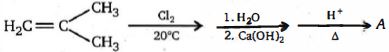
- 1View Solutionનીચેની પ્રક્રિયા દરમિયાન મુખ્ય નીપજ કઈ હશે ?
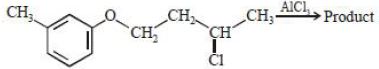
- 2View Solutionઇથાઇનની સમાનધર્મીં શ્રેણીમાં આવતું સંયોજન......... છે.
- 3સંયોજનો $A$ અને $B$ નીચેની પ્રક્રિયા માટે અનુક્રમે શોધો?View Solution
બેન્ઝિન $\xrightarrow{HCHO+HCl}\,A$$\xrightarrow{AgCN}\,B$
- 4નીપજ $(C)$ શોધોView Solution
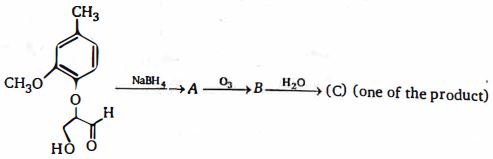
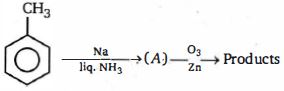
- 5View Solutionનીચેનામાંથી કઈ નીપજ ઉપરોક્ત પ્રક્રિયામાંથી મેળવી શકાતી નથી ?
- 6નીચે આપેલ પ્રક્રિયામાં $X$ એ શું હશે?View Solution
$C{H_3}C{H_2}CH = CHC{H_3}$ $\xrightarrow{X}C{H_3}C{H_2}COOH\, + \,C{H_3}COOH$
- 7$(C_5H_9Cl)$ નો પ્રકાશિય સમઘટક $(A)$ તેની પ્રકિયા હાઈડ્રોજન ના એક મોલ આપે છે. નિષ્ક્રિય પ્રકાશક્રિયાશીલ સંયોજન $(B)$ સંયોજન $(A)$ શું હશે ?View Solution
- 8$CH_3CO_3H$ સાથે મિથાઇલ સાયક્લોપેંટીન એ $1$ -મિથાઇલ સાયકલોપેન્ટેનની પ્રક્રિયા દરમિયાન બનાવેલ મુખ્ય નીપજ શું છે ?View Solution
- 9View Solutionનીચેનામાંથી કોનો ઓક્ટેન આંક શૂન્ય છે ?
- 10$A$..... શોધોView Solution