The reaction occurs at standard hydrogen electrode $(S.H.E)$ is given below :
$H _{2} \rightarrow 2 H ^{+}+2 e ^{-}$
At $1\,atm$ pressure, $pH$ is equal to $10$ .
Therefore,
$\left[ H ^{+}\right]=10^{-10}$
The standard reduction potential of $S.H.E$ is equal to zero.
Apply Nernst equation,
$E_{ H _{2} / H ^{+}}=0-\frac{0.059}{2} \log \frac{\left(10^{-10}\right)^{2}}{1}$
$=0.0295 \times 20$
$=0.59 \,V$
Download our appand get started for free
Similar Questions
- 1$25^o$ સે.પ્રક્રિયાનો પ્રમાણિત રીડક્શન પોટેન્શિયલ નીચે મુજબ છે. કયો પ્રબળ રીડ્યુસીંગ કર્તા છે?View Solution
$Zn^{2+}(aq) + 2e^{-} $ $\rightleftharpoons$ $ Zn (s)$ , $E^o_{RP}= -0.762\, V$,
$Cr^{3+}(aq) + 3e^{-} $ $\rightleftharpoons$$ Cr(s)$, $E^o_{RP} = -0.740\, V$
$2H^{+}(aq) + 2e^{-} $$\rightleftharpoons$$ H_2(g)$, $E^o_{RP} = 0.00\,V$,
$Fe^{3+}_{(aq)} + 2e^{-} $$\rightleftharpoons$$ Fe^{2+}(aq)$ , $E^o_{RP} = 0.77 \,V$
- 2નીચે આપેલા અર્ધકોષોથી બનતા કોષનો $EMF$ કેટલા ............ $\mathrm{V}$ થશે?View Solution
$M{g^{2 + }} + 2{e^ - } \to Mg(s);\,\,E = - 2.37\,V$
$C{u^{2 + }} + 2{e^ - } \to Cu(s);\,\,\,E = + 0.33\,V$
- 3એલ્યુમિનિયમ ક્લોરાઈડના દ્રાવણ એલ્યુમિનિયમનો $1$ ગ્રામ પરમાણુ (પરમાણુ ભાર $=27$ ) પ્રાપ્ત કરવા માટે ............ $\mathrm{N}$ ઇલેક્ટ્રોનોની જરૂર પડશે. (જ્યાં, $N$ એવોગેડ્રો આંક છે)View Solution
- 4નીચેની પ્રક્રિયા માટે કોષનો $ emf$ આપેલ છે. $Zn (s) + Ni^{2+}_{(aq)}\, (a = 0.1) \rightarrow Zn^{2+}_{(aq)}\, (a = 1.0) + Ni\,\, 298\,K$. એ $0.5105\, V$ જોવા મળે છે કોષમાં પ્રમાણિત $e$ કેટલા. ................ $\mathrm{V}$ છે?View Solution
- 5$298 \mathrm{~K}$. પર કોષ (image) ના વડે તેને વધારી શકાય છે.View Solution
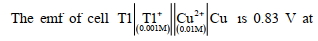
- 6$298$ $K$ પર આપેલ અર્ધ કોપ માટે પોટેન્શિયલ (-)_________ $\times 10^{-2} \mathrm{~V}$ છે.View Solution
$2 \mathrm{H}_{(\mathrm{aq})}^{+}+2 \mathrm{e}^{-} \rightarrow \mathrm{H}_2(\mathrm{~g})$
${\left[\mathrm{H}^{+}\right]=1 \mathrm{M}, \mathrm{P}_{\mathrm{H}_2}=2 \mathrm{~atm}}$
(Given: $2.303 \mathrm{RT} / \mathrm{F}=0.06 \mathrm{~V}, \log 2=0.3$ )
- 7$Zn \rightarrow Zn^{+2} + 2e^{-}$ અર્ધપ્રક્રિયા માટેના પ્રમાણિત ઑક્સિડેશન પોટૅન્શિયલનાં મૂલ્યો આ પ્રમાણે આપેલ છે : $E^{0}_{Oxi} = +0.76\,V$, $Fe \rightarrow Fe^{2+} + 2e^{-}$ , $E^{0}_{Oxi} = +0.41\,V$. $Fe^{2+} + Zn \rightarrow Zn^{2+} + Fe$ પ્રક્રિયા માટે કોષનો પોટૅન્શિયલ ............ $\mathrm{V}$ શોધો.View Solution
- 8$NaI, NaNO _{3}$ અને $AgNO _{3}$ ની સિમિત મોલર વાહકતાઓ (limiting molar conductivities) અનુક્રમે $12.7,12.0$ અને $13.3\, mS m { }^{2}\, mol ^{-1}$ છે. (બધા $25^{\circ} C$ તાપમાને). તો આ જ તાપમાને $AgI$ ની સિમિત મોલર વાહકતા $......\,mS m ^{2}\, mol ^{-1}$ છેView Solution
- 9$18$ ગ્રામ $ H_2O$ ના વિદ્યુત વિભાજ્યના વિઘટન માટે $ 3 $ એમ્પિયર પ્રવાહ કેટલા ........... કલાક સમય સુધી પસાર કરવામાં આવે છે?View Solution
- 10તત્વ $I, II, III$ અને $IV$ ના $E^{0}_{Red} $ નાં મૂલ્યો અનુક્રમે $-3.04 \,V, -1.90\, V, 0.00 \,V, 1.90\, V$ હોય, તો મહત્તમ રિડકશનકર્તા તરીકેની ક્ષમતા ધરાવતું તત્વ ..... હશે.View Solution
