$300 \,K$ અને $1 \,atm$ દબાણ પર $15\, mL$ વાયુમય હાઇડ્રોકાર્બનના પૂર્ણ દહન માટે $375 \, mL$ વાયુ જેમાં હવામાં $ 20 \%\, \, O_2 $ ઓક્સિજનની માત્રા જરૂરી છે.દહન પછી વાયુઓ $330 \, mL$ ધારણ કરે છે. ધારો કે રચાતું પાણી પ્રવાહી સ્વરૂપમાં છે અને કદ સમાન તાપમાન અને દબાણ પર માપવામાં આવ્યું હતું, તો હાઇડ્રોકાર્બનનું સૂત્ર શું છે?
JEE MAIN 2016, Diffcult
d
\(C_{x} H_{y(g)}+\left(\frac{4 x+y}{4}\right) O_{2(g)} \rightarrow x C O_{2(g)}+\frac{y}{2} H_{2} O(l)\)
\(C_{x} H_{y(g)}+\left(\frac{4 x+y}{4}\right) O_{2(g)} \rightarrow x C O_{2(g)}+\frac{y}{2} H_{2} O(l)\)
Volume of \(O_{2}\) used
\(=375 \times \frac{20}{100}=75 m l\)
From the reaction of combination
\(m l C_{x} H_{y}\) requires \(=\frac{4 x+y}{4} m l O_{2}\)
\(15\left(\frac{4 x+y}{4}\right)=75\)
So, \(4 x+y=20\)
\(x=3\)
\(y=8\)
\(\therefore C_{3} H_{8}\)
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1View Solutionઇથેનના અણુકોણાત્મક સમઘટકોના સંદર્ભમાં નીચેના પૈકી ક્યુ વિધાન સાચું છે ?
- 2View Solutionપ્રકિયા ની મુખ્ય નીપજ કઈ હશે ?
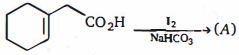
- 3એક આલ્કાઇલ બ્રોમાઇડ $(X)$ ની $Na$ સાથેની પ્રક્રિયાથી $4, 5-$ ડાયઇથાઇલ ઓક્ટેન મળે છે. તો $X$............. થશે.View Solution
- 4નીચે આપેલ પ્રક્રિયાઆની શ્રેણીમાં મુખ્ય નીપજ શું થશે $?$View Solution
$n - Bu -\equiv\frac{(i) n-BuLi,n - C _{5} H _{11} Cl}{(ii) Lindlar\,\, cat, H _{2}}$
- 5............ ઓલેફિનના ઓઝોનોલિસિસથી $CH_3CH_2CHO$ અને $CH_3CHO$ મળે છે.View Solution
- 6$p-$ નાઇટ્રોફિનોક્સાઇડ આયનનું સંસ્પંદન બંધારણની સૌથી અસંભવિત રજૂઆત કઈ છે?View Solution
- 7View Solutionનીચે આપેલી પ્રકિયા ની નીપજ શું હશે ?
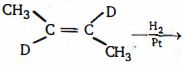
- 8બેન્ઝિનમાં બધા જ કાર્બનો વચ્ચે $C - C$ બંધ લંબાઈ સમાન છે કારણ કે...View Solution
- 9View Solutionઓછા કાર્બન નંબરના હાઇડ્રોકાર્બન તૈયાર કરવાની સૌથી મહત્વપૂર્ણ પદ્ધતિ કઈ છે?
- 10View Solutionચોક્કસ આલ્કેનને ક્લોરીન સાથે મિશ્ર કરવામાં આવે છે. પારજાંબલી પ્રકાશ સાથે પ્રકિરણન થાય છે. તે માત્ર એક મોનોક્લોરોઆલ્કેન આપે છે. તો આ આલ્કેન કયો છે ?
