$85.8\%$ કાર્બન ધરાવતા એક હાઇડ્રોકાર્બન $A$ ના પ્રતિ અણુ હાઈડ્રોજન પરમાણુઓની સંખ્યા $........$ છે.(આપેલ : $A$ નું મોલર દળ = $84\,g\,mol ^{-1}$ )
JEE MAIN 2023, Medium
d
| Element | Percentage | Mole | Mole ratio |
| \(C\) | \(85.8\) | \(\frac{85.8}{12}=7.15\) | \(1\) |
| \(H\) | \(14.2\) | \(\frac{14.2}{1}=14.2\) | \(2\) |
Empirical formula \(\left( CH _2\right)\)
\(14 \times n=84\)
\(n =6\)
\(\therefore\) Molecular formula \(C _6 H _{12}\)
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1પ્રકિયા $CH_2 = CH_2$ $\xrightarrow[{acid}]{{Hypochlorous}}M\xrightarrow{R}$ $\begin{array}{*{20}{c}}View Solution
{C{H_2}OH} \\
{|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,} \\
{C{H_2}OH}
\end{array}$ માં $M$ અને $R$ અનુક્રમે ........ થશે. - 2આલ્કીન સાથે $HCl$ની પ્રક્રિયા થઈને નીપજ $1-$ક્લોરો${-1}$મિથાઇલસાયકલોહેકઝેન આપવાના માર્કોવનિકોવના નિયમ અનુસાર પ્રતિક્રિયા આપે છે, માટે શક્ય આલ્કીન શું હશે?View Solution
- 3આલ્કોક્સિમર્ક્યુરેશન ડી-મર્ક્યુરેશન ની નીપજ $(A)$ ........છેView Solution
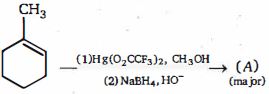
- 4View Solutionમિથાઇલ મેગ્નેશિયમ બ્રોમાઇડની ઇથાઇલ આલ્કોહોલ સાથેની પ્રક્રિયાથી મળતી નીપજ .......છે.
- 5$X $ ઉપર ઓઝોનોલીસીસ કરતા અને પછી રીડકશન કરતા બે મોલ સમાન આલ્ડીહાઈડ આપે છે તો $ 'X'$ શું હશે ?View Solution
- 6પેરોક્સાઇડની હાજરીમાં પ્રોપિનની પ્રક્રિયા $HBr$ સાથે કરતાં મળતી નીપજ...... છે.View Solution
- 7નીચેનામાંથી સંયોજનને $(3)$ બાકીના સંયોજનોમાંથીઅલગ પાડવા માટે કયો સૌથી યોગ્ય પ્રક્રિયક છે ?View Solution
$1.\,\,CH_3-C \equiv C -CH_3$
$2.\,\,CH_3 - CH_2 - CH_2 - CH_3$
$3. \,\,CH_3 - CH_2C \equiv CH$
$4.\,\,CH_3 - CH = CH_2$
- 8નીચેનામાંથી કયું વિધાન એ $A$ અને $B$ માટે સાચું છે ?View Solution
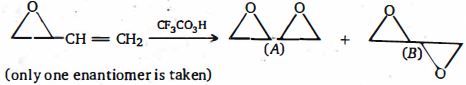
- 9નીચેનામાંથી કયું એ સિસ અને ટ્રાન્સ - $1,4$ -ડાયમીથાઇલ સાયકલોહેકઝેન નું મિશ્રણ આપશે.જ્યારે ઉત્પ્રેરક હાઈડ્રોજીનેશન થાય છેView Solution
- 10View Solutionજ્યારે ટોલ્યુઈનને પ્રકાશની હાજરીમાં અને હેલોજન કેરીયરની ગેરહાજરીમાં ક્લોરીન સાથે ગરમ કરવામાં આવે તો શું ઉત્પન્ન થાય ?
