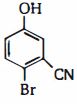
આ સંયોજન નું $IUPAC$ નામ શું હશે ?

Medium
b
In this compound, the first priority is given to the \(- CN\) functional group. So, the numbering will start from carbon attached to \(- CN\) group.
In this compound, the first priority is given to the \(- CN\) functional group. So, the numbering will start from carbon attached to \(- CN\) group.
Now, the lowest set of locant rule is considered.
Number \(1\) is given to \(- CN\) group, \(- Br\) will be at the \(2^{\text {nd }}\) position and \(- OH\) will be at the \(5^{\text {th }}\) position.
So, the name is \(2\)-bromo-\(5\)-hydroxybenzonitrile.
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1${H_3}C - \mathop C\limits_{\mathop |\limits_{{C_2}{H_5}} } H - \mathop C\limits_{\mathop |\limits_{{C_2}{H_5}} } H - C{H_3}$ પદાર્થનું ${\text{IUPAC}}$ નામ ક્યું છે?View Solution
- 2નીચેના સંયોજનનું $IUPAC$ નામ શું છે?View Solution
- 3પદાર્થનું $IUPAC$ નામ જણાવો.View Solution

- 4નીચેના સંયોજનમાં કેટલા $(i)$ $sp ^{2}$ સંકૃત કાર્બન પમાણુઓ અને $(ii)$ $\pi$ બંધ હાજર છે ?View Solution
- 5આપેલ પદાર્થમાં શૃંખલા માં કાર્બન પરમાણુની સંખ્યા કેટલી છે?View Solution
$C{H_3}\, - \,\,C{H_2}\, - \,\,C{H_2}\, - \,\,\mathop C\limits_{\mathop {||}\limits_{OHC\,\, - \,\,C\,\, - \,\,C{H_2}\, - \,\,C{H_3}} } \,\, - \,\,COOH$
- 6$C_5H_{10}O$ અણુસૂત્રમાં આલ્ડીહાઈડની કુલ સંખ્યા કેટલી?View Solution
- 7સકસિનિક એસિડનું $IUPAC$ નામ શું છે?View Solution
- 8એક કાર્બનિક સંયોજનના નામમાં નીચે આપેલા ક્રિયાશીલ સમુહની પ્રાથમિકતાનો સાચો ધટતો કમ તેના $IUPAC$ નામકરણની પ્રણાલીને આધારીત કરો.View Solution
- 9પદાર્થનું $IUPAC$ નામ જણાવો.View Solution
- 10$2, 3-$ડાઈહાઈડ્રોક્સિ બ્યુટેનડોઈક એસિડનું મૂળ નામ શું છે ?View Solution
