Download our appand get started for free
Similar Questions
- 1View Solutionઇથિલીન, કાર્બન મોનોક્સાઇડ અને પાણીના મિશ્રણને ઊચા તાપમાને ગરમ કરતાં શુ મળે છે ?
- 2View Solutionઆલ્કાઇન સંયોજનો....... પ્રક્રિયા દર્શાવી શકે.
- 3View Solutionનીચેનામાંથી કયા આલ્કોહોલ એ આલ્કિન ના હાઇડ્રેશનમાંથી બનાવી શકાતા નથી?
- 4View Solutionનાઇટ્રોબેન્ઝિન ના નાઇટ્રેશનથી ..... બને છે.
- 5નીચેની પ્રક્રિયાઓ માટે મુખ્ય નિપજો બતાવવામાં આવે છેView Solution
${H_2}C = CH - CH = C{H_2}\xrightarrow[{0{\,^o}C}]{{HBr}}$ $\begin{array}{*{20}{c}}
{{H_2}C = CH - CH - C{H_3}} \\
{\,\,\,\,\,\,\,\,\,\,\,|} \\
{\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,Br\,\,\,\,\,\,}
\end{array}\xrightarrow{{ + 25{\,^o}C}}$ $\begin{array}{*{20}{c}}
{C{H_2}CH = CHC{H_3}} \\
{|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,} \\
{Br\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}
\end{array}$આ $......1......$ નીચા તાપમાને નિયંત્રણ અને $......2......$ ઉચ્ચ તાપમાન પર નિયંત્રણનું ઉદાહરણ પ્રદાન કરે છે.
- 6નીચેના પૈકી ક્યા આલ્કીનની જ્યારે $HCI$ સાથે પ્રક્રિયા કરવામાં આવે ત્યારે મુખ્યત્વે પ્રતિમાર્કોવનિકોવ નીપજ આપે છે?View Solution
- 7View Solutionનીચે આપેલ સંયોજનો પૈકી કોઈ એક કે જે સૌથી વધારે દ્વિધ્રુવ ચાકમાત્રા પ્રદર્શિત કરે છે તે શોધો.
- 8View Solutionજ્યારે સાયકલો હેકઝેનને પાણીમાં નાખતા તે તરે છે કારણ કે.....
- 9${{\text{C}}_{\text{6}}}{{\text{H}}_{\text{6}}}\text{ + CO + HCl }\,\xrightarrow{\text{A}}\,{{C}_{6}}{{H}_{5}}CHO\,\,+\,\,HCl\,$ ઉપર ની પ્રક્રિયામાં $\text{ }\!\!'\!\!\text{ A }\!\!'\!\!\text{ = }.......$View Solution
- 10નીચે બે વિધાનો આપેલા છે. એકને કથન $A$ અને બીજાને કારણ $R$ વડે લેબલ કરેલ છે.View Solution
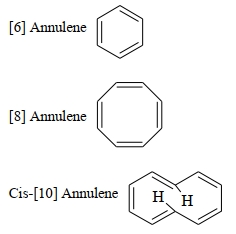
કથન $A$ : $[6]$ એન્યુલીન, $[8]$ એન્યુલીન, સિસ-$[10]$ એન્યુલીન અને ટ્રાન્સ -$[10]$ એન્યુલીન ક્રમશઃ એરોમેટિક, નોન-. એરોમેટિક, એરોમેટિક અને નોન-એરોમેટિક છે
કારણ $R$ : એરોમેટિક અને એન્ટી એરોમેટિક પ્રણાલી માટે સમતલીયતા એ એક જરૂરિયાત છે.
ઉપરોક્ત વિધાનોના સંદર્ભે આપેલા વિકલ્પોમાંથી યોગ્ય ઉત્તર પસંદ કરો