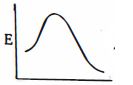
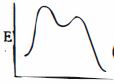
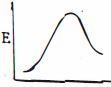
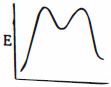
આપેલ પ્રક્રિયાને કઇ ઉર્જા આકૃતિ શ્રેષ્ઠ રજૂ કરે છે?
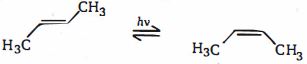
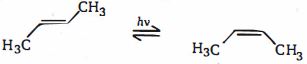
Diffcult
d
\((d)\) It is possible to interconvert cis and trans alkenes, but the \(\pi\) -bond must be broken first. This requires a considerable amount of energy - around \(260\, kJ\, mol^{-1}\) .One way to break the \(\pi\) -bond would be to promote an electron from the \(\pi\) -orbital to the \(\pi^*\) -orbital. If this were to happen, there would be one electron in the bonding \(\pi\) -orbital and one in the antibonding \(\pi^*\) -orbital and hence no overall bonding. Electromagnetic radiation of the correct energy could promote the electron from \(HOMO\) to \(LUMO\). The correct energy actually corresponds to light in the ultraviolet \((UV)\) region of the spectrum. Thus, shining \(UV\) light on an alkene would promote an electron from its bonding \(\pi\) -molecular orbital to its antibonding \(\pi^*\) -molecular orbital, thereby breaking the \(\pi\) -bond (but not the \(\sigma\) - bond) and allowing rotation to occur.
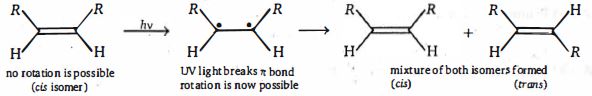
\((d)\) It is possible to interconvert cis and trans alkenes, but the \(\pi\) -bond must be broken first. This requires a considerable amount of energy - around \(260\, kJ\, mol^{-1}\) .One way to break the \(\pi\) -bond would be to promote an electron from the \(\pi\) -orbital to the \(\pi^*\) -orbital. If this were to happen, there would be one electron in the bonding \(\pi\) -orbital and one in the antibonding \(\pi^*\) -orbital and hence no overall bonding. Electromagnetic radiation of the correct energy could promote the electron from \(HOMO\) to \(LUMO\). The correct energy actually corresponds to light in the ultraviolet \((UV)\) region of the spectrum. Thus, shining \(UV\) light on an alkene would promote an electron from its bonding \(\pi\) -molecular orbital to its antibonding \(\pi^*\) -molecular orbital, thereby breaking the \(\pi\) -bond (but not the \(\sigma\) - bond) and allowing rotation to occur.

Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1View Solutionનીચેનામાંથી કયા સંયોજન માંઇલેક્ટ્રોનઅનુરાગી ચક્રીય વિસ્થાપન ડાબી બાજુ હાજર ફિનાઇલ રિંગમાં થાય છે
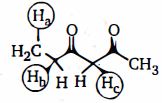
- 2હાઈડ્રોજન અણુઓને ક્રમ આપો $(H_a, H_b, H_c)$ તેમની એસિડિક પ્રબળતાના ક્રમમાં નીચેના કયા અણુમાં હાજર છે.View Solution
- 3View Solutionનીચે પૈકી કોની દ્વિધ્રુવીય ચાકમાત્રા સૌથી વધારે છે?
- 4View Solutionનીચેનામાંથી કોની દ્વિધ્ર્રુવીય ચાકમાત્રા શૂન્ય થશે ?
- 5આપેલા પરમાણુના નામાંકનમાં, $H$ અણું શામેલ છેView Solution
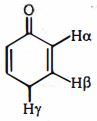
- 6View Solutionનીચે આપેલ જુથ માંથી મેટા નિર્દેશક ક્રિયાશીલ સમૂહોનું જૂથ શોધો.
- 7આયનીકરણની સ્થિરતાનો ક્રમ કયો છે?View Solution
${\rm{(X)}}{\rm{. }}C{H_3} - \,\,\,\mathop C\limits^ \oplus H\,\, - \,\,C{H_3}$
${\rm{(Y)}}{\rm{. }}C{H_3} - \,\,\,\mathop C\limits^ \oplus H\,\, - \,\,O\,C{H_3}$
${\rm{(Z)}}{\rm{. }}C{H_3} - \,\,\,\mathop C\limits^ \oplus H\,\, - \,\,CO\,C{H_3}$
- 8નિક્ટતમ પરમાણુ પર હાજર અબંધકારક ઈલેક્ટ્રોન યુગ્મ અને $\pi$ બંધ વચ્ચેની પારસ્પરિક ક્રિયા માટે જવાબદાર_____View Solution
- 9View Solutionસ્થાયી કાર્બોકેટાયન જે ઉપરની પ્રક્રિયામાં બને છે તે શોધો.
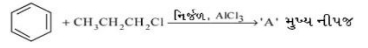
- 10View Solutionસૌથી સ્થિર કાર્બએનાયન કયો છે ?