આપેલા સંયુગ્મ બેઇઝની વધતી બેઝિકતાનો સાચો ક્રમ થશે.$( R = CH_3)$
AIEEE 2010, Medium
d
Conjugate acid base pair - If bronsted acid is a strong acid then its conjugate base is a weak base and vice versa.
Conjugate acid base pair - If bronsted acid is a strong acid then its conjugate base is a weak base and vice versa.
$H C l+H_{2} O \rightleftharpoons H_{3} O^{+}+C l^{-}$
$\mathrm{Cl}^{-}$ is conjugate base of $\mathrm{HCl} \& \mathrm{H}_{3} \mathrm{O}^{+}$ is conjugate acid of $\mathrm{H}_{2} \mathrm{O}$
Stronger acids has weaker conjugate base aciding order:
$R C O O H>H C \equiv C H>N H_{3}>R H$
So basicity order : $R C O O^{-} < H C \equiv C^{-} < N H_{2}^{-} < R^{-}$
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1નીચેના માટે એસિડિક ક્ષમતાનો સાચો ક્રમ લખોView Solution
$(I)$ $ CH_3 - NO_2$
$(II)$ $NO_2 - CH_2 - NO_2$
$(III)$ $ CH_3 - CH_2 - NO_2 $
$(IV)$ $\begin{array}{*{20}{c}}
{N{O_2} - CH - N{O_2}} \\
{|\,\,\,\,\,\,} \\
{\,N{O_2}}
\end{array}$ - 2View Solutionઆપેલ કઈ જોડીઓમાં, જેમાં જોડીનું બીજું સંયોજન પ્રથમ કરતા વધુ એસિડિક છે
- 3View Solutionનીચે આપેલા માટે સૌથી વધુ સ્થાયી કાર્બોકેશાયન શોધી.
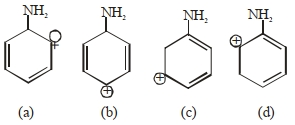
- 4View Solutionનીચેના પૈકી કયું સંયોજન સૌથી વધારે એસિડિક છે?
- 5View Solutionઆપેલ સંયોજન માં મોટાભાગના એસિડિક હાઇડ્રોજનને ઓળખો.
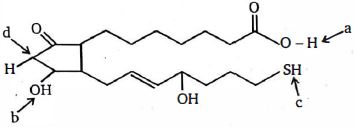
- 6View Solutionનીચેનામાંથી કયો સૌથી બેઝિક પદાર્થ છે ?
- 7View Solutionનીચેનામાંથી કયો જલીય માધ્યમમાં પ્રબળ બેઈઝ છે ?
- 8View Solutionનીચેનામાંથી કયો પદાર્થ હાઈડ્રોકઝીલીક પ્રોટોનથી સૌથી એસિડિક છે ?
- 9તે સ્થળ ઓળખો, જ્યાં $H^+$ નો હુમલો સૌથી અનુકૂળ છેView Solution
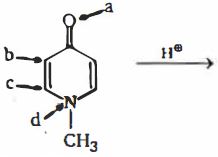
- 10View Solutionનીચેનામાંથી કયું સંયોજન ધન મેસોમેરિક અસર દર્શાવે છે ?
