બેન્ઝાઇલ કાર્બોનિયમ આયન (image) ની સંકરણ અવસ્થા શું છે?
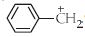

NEET 2013, Medium
a
All carbocations are \(sp^2\) hybridised and they have planar triangular structure. The value of the \(\mathrm{H}=3\) only.
All carbocations are \(sp^2\) hybridised and they have planar triangular structure. The value of the \(\mathrm{H}=3\) only.
\(\mathrm{H}=\frac 12[\) Valence electron of the central atom \(+\) no of surrounding atomcation \(+\) anion]
\(=\frac 12[4+3-1+0]\)
\(=6 / 2=3\)
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1$2-$ઓકસીહેકઝ$-4-$આઇનોઇક એસિડ માં 'સિગ્મા'અને $'Pi'$ બંધો ની કુલ સંખ્યા .......... છે.View Solution
- 2$\begin{array}{*{20}{c}}View Solution
{C{H_3} - CH - CH - CH - C{H_3}} \\
{\,|\,\,\,\,\,\,\,\,\,\,\,\,\,\,|\,\,\,\,\,\,\,\,\,\,\,\,\,|\,\,\,\,\,} \\
{Cl\,\,\,\,\,\,\,\,\,Br\,\,\,\,\,\,\,\,\,OH}
\end{array}$ નું $IUPAC$ નામ શું હશે ? - 3${(C{H_3})_3}C - CH = C{H_2}$નું $IUPAC$ નામ શું હશે?View Solution
- 4નીચે પૈકી કયું સંયોજન જે કાર્બન બંધ બનાવવા માટે માત્ર $s{p^3}$ સંકૃત કક્ષકનો ઉપયોગ કરે છે.View Solution
- 5$C{H_3}\, - \,\,\mathop C\limits^{\mathop |\limits^{COOH} } H\,\, - \,\,C{H_2}\, - \,\,\mathop C\limits^{\mathop |\limits^{COOH} } H\,\, - \,\,\mathop C\limits^{\mathop |\limits^{C{H_3}} } H\,\, - \,\,COOH$ નું નામ જણાવોView Solution
- 6સંયોજન $CH_3-CH(CH_3)-COCH_3 $ નું $ IUPAC$ નામ ...View Solution
- 7ટ્રાન્સ$-2-$હેકઝેનાલનું સાચું બંધારણ કયું છે ?View Solution
- 8પદાર્થનું $IUPAC$ નામ શું છે ?View Solution
- 9$\begin{matrix}View Solution
C{{H}_{3}}\,\,\, \\
|\,\,\,\,\,\,\,\,\,\,\, \\
C{{H}_{2}}=C-COOC{{H}_{3}} \\
\end{matrix}$ નું $IUPAC$ નામ શું હશે ? - 10$2-$ઓકસીહેકઝ$-4-$આઇનોઇક એસિડ માં 'સિગ્મા'અને $'Pi'$ બંધો ની કુલ સંખ્યા .......... છે.View Solution
