બેન્ઝિનમાં પ્રત્યેક કાર્બન-કાર્બન બંધનો બંધ-ક્રમાંક શું છે?
IIT 1981, Medium
c
The bond order of individual carbon-carbon bonds in benzene is between one and two.It is due to resonance structure of \(C _6 H _6\). In the benzene molecule, carbon atoms form a ring with alternating single and double bonds in between each of them. Each carbon atom forms one bond with one carbon atom and one bond with another. The bonding electrons are delocalized over the entire molecule. Thus, benzene is a resonance hybrid of two equivalent structures, and the single and double bonds oscillate from one position to the other.
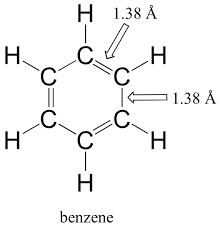
The bond order of individual carbon-carbon bonds in benzene is between one and two.It is due to resonance structure of \(C _6 H _6\). In the benzene molecule, carbon atoms form a ring with alternating single and double bonds in between each of them. Each carbon atom forms one bond with one carbon atom and one bond with another. The bonding electrons are delocalized over the entire molecule. Thus, benzene is a resonance hybrid of two equivalent structures, and the single and double bonds oscillate from one position to the other.

Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1$n - Butane \xrightarrow{{C{l_2}/hv}}$View Solution
મોનોક્લોરો ઉત્પાદનોની કુલ સંખ્યા આપો (અવકાશીય સમઘટકતા સહિત), જે ઉપરોક્ત પ્રતિક્રિયામાં કેટલા શક્ય છે.
- 2જ્યારે ઓક્સિડેટીવ ઓઝોનોલિસિસમાંથી પસાર થાય છે ત્યારે કયું સંયોજન એ $CO_2$ ગેસ મુક્ત કરતો નથી ?View Solution
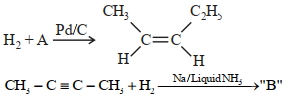
- 3આપેલ પ્રક્રિયામાં $A$ અને $B$ ઓળખો.View Solution
- 4View Solutionસ્થિરતાનો સાચો ક્રમ કયો છે ?
- 5નીચે આપેલ પ્રક્રિયામાં $X$ એ શું હશે?View Solution
$C{H_3}C{H_2}CH = CHC{H_3}$ $\xrightarrow{X}C{H_3}C{H_2}COOH\, + \,C{H_3}COOH$
- 6નીચેનામાંથી જે સૌથી મજબૂત $o, p-$ નિર્દેશક સમૂહ છે .....View Solution
- 7ઉપરોક્ત સંયોજનોના $o-$ નાઈટ્રેશનનો દર, $(I)$ ટોલ્યુઇન, $(II)\, 2-D-$ ટોલ્યુઇન અને $(III) \,2, 6-D_2$ --ટોલ્યુઇન છે નીચેના ક્રમમાં કયો છેView Solution
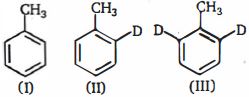
- 8પ્રોપાઈનના દ્વિ-બંધ પર $ HI$ ને ઉમેરતા આઈસો-પ્રોપાઈલ આયોડાઈડ મળે છે અને $n$-પ્રોપાઈલ આયોડાઈડ મળતા નથી, કારણ તેનાં.......દ્વારા ઉમેરવાની પ્રક્રિયા થાય છે.View Solution
- 9$1-$ butene સાથે હાઇડ્રોજન ક્લોરાઇડની પ્રક્રિયાના પ્રથમ તબ્બકા નીચેનામાંથી ક્યા સચોટ રીતે વર્ણવે છે?View Solution
- 10બેન્ઝિનમાં $C - C$ બંધની લંબાઇ ......$\mathop A\limits^o $ છે.View Solution
