Download our appand get started for free
Similar Questions
- 1$ 1-$ બ્યુટાઈનના ઓક્સિમરક્યુરેશન $(HgS{O_4} + {H_2}S{O_4})$ દ્વારા મેળવેલ નીપજ (ઓ) કઈ હશે?View Solution
- 2View Solutionપ્રોપાઈનના હાઈડ્રેશનના પરિણામે.......નું નિર્માણ થાય છે.
- 3નીચેનામાંથી કયો $ o^-/p^- $ નિર્દેંશિત સમૂહ છે ?View Solution
- 4નીચેનામાંથી કયા કાર્બનિક ક્લોરાઇડ્સ બેન્ઝીન અને $AlCl_3$સાથે ગરમ થાય ત્યારે ફ્રિડેલ-ક્રાફ્ટ આલ્કાઇલલેશન નીપજ આપશે નહીંView Solution
- 5View Solutionઆ પ્રકિયા ની નીપજ શું હશે ?
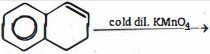
- 6નીચેની પ્રક્રીયાને ધ્યાનમાં લો. કઈ પ્રક્રીયા મુક્ત મુલક ક્રિયાવિધીથી પૂર્ણ થાય છે?View Solution
(a) $C{H_3}\, - \,\,\mathop C\limits_{\mathop |\limits_{C{H_3}} } \, = \,\,C{H_2}\,\xrightarrow{{HBr}}\,\,C{H_3}\, - \,\,\mathop {\mathop C\limits_{\mathop |\limits_{C{H_3}} } }\limits^{\mathop |\limits^{Br} } \,\, - \,\,C{H_3}$
(b) $C{H_3}\, - \,\,CH\,\, = \,\,C{H_2}\,\mathop {\xrightarrow{{HBr}}}\limits_{Peroxide} \,\,C{H_3}\,C{H_2}\,C{H_2}\,Br$
(c) $C{H_4}\, + \,\,C{l_2}\,\xrightarrow{{hv}}\,\,C{H_3}Cl\,\, + \,\,HCl$
- 7View Solutionનીચેની પ્રકિયામાં નીપજ.......
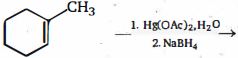
- 8બ્રોમિનેશનની પ્રક્રિયામાં $1\,^oH, 2\,^oH$ અને $3\,^oH$ માં પ્રક્રિયાશીલતાનો પ્રક્રિયા દર અનુક્રમે $1 : 82 : 1600$ છે તો પ્રક્રિયામાં $ (a)$ અને $(b)$ નીપજ કેટલી ટકાવારીમાં ઉપજ મળશે ?View Solution
$C{{H}_{3}}\,-\,\,\overset{\overset{C{{H}_{3}}}{\mathop{|}}\,}{\mathop{C}}\,H\,\,-\,\,C{{H}_{3}}(excess)\,\,+\,\,B{{r}_{2}}\,\xrightarrow{hv}$
$\,\,\,\,C{{H}_{3}}\,-\,\,\overset{\overset{C{{H}_{3}}}{\mathop{|}}\,}{\mathop{\underset{\underset{Br}{\mathop{|}}\,}{\mathop{C}}\,}}\,\,\,-\,\,C{{H}_{3}}\,+\,\,C{{H}_{3}}\,-\,\,CH\,\,-\,\,C{{H}_{2}}\,-\,\,Br$
- 9View Solutionનીચેનામાંથી કયું સંયોજન સંસ્પંદન દર્શાવતું નથી?
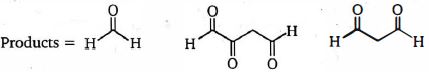
- 10View Solutionનીચેના ત્રણ ઉત્પાદનો આપવા માટે ટ્રાઇન ને એસીટીકએસિડમાં ઝીંક પછી ઓઝોન દ્વારા સારવાર આપવામાં આવે છે. તો ટ્રાઈન ની રચના શું છે?