$BF_2NH_2$ પરમાણુ સંબંધિત ખોટું વિધાન ક્યું છે?
Advanced
d
\(*\) Both boron and nitrogen are \(sp^2-\) hybridised.
\(*\) Both boron and nitrogen are \(sp^2-\) hybridised.
\(*\, FBF\) bond angle \(< 120^o\) (\(VSPER\) theory)
\(* \,H N H\) bond angle is less than \(120^o\) but greater than \(109^o28\)' due to back bonding.
\(*\) Due to presence of \(H-\) atom attached to nitrogen this molecule can exhibits intermolecular \(H-\) bonding.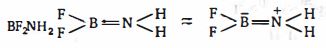
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1નીચેના માંથી ક્યાં સમુહ માં $sp^3$ સંકરણ જોવા મળતુ નથીView Solution
- 2$M$ ધાતુની ઇલેક્ટ્રોન રચના $1s^2 \,2s^2\,2p^6\,3s^1$ છે, તો તેના ઓક્સાઇડનું સૂત્ર શુ થશે ?View Solution
- 3નીચે પૈકી કયા સમઇલેક્ટ્રોનિય અને સમબંધારણીય છે?View Solution
$NO^-_3, CO^{2-}_3 ,CO^-_3 ,SO_3$
- 4View Solutionસલ્ફયુરિક એસિડ અણુ ધરાવે છે .......
- 5સલ્ફર ક્લોરિન સાથે $1 : 2$ ગુણોત્તરમાં પ્રક્રિયા આપે છે અને $X$ બનાવે છે. $X$નું હાઇડ્રોલિસિસ સલ્ફર સંયોજન $Y$ આપે છે.$Y$ની આયનની બંધારણ અને સંકરણ શું છે?View Solution
- 6વિધાન :પરમાણુ નાઇટ્રોજન પરમાણુ ઓક્સિજન કરતા ઓછા પ્રતિક્રિયાશીલ હોય છે.View Solution
કારણ : $N_2$ ની બંધ લંબાઈ ઑક્સિજન કરતા ટૂંકા હોય છે - 7View Solutionનીચે પૈકી કયું સંસ્પંદન રચનાઓ વિષે સાચું નથી?
- 8$N{H_3}$, ${[PtC{l_4}]^{2 - }},\,PC{l_5}$ અને $BC{l_3}$ આપેલા ઘટકો માં કેન્દ્રિય અણુના સંકરણ નો સાચો ક્રમ કયો હશે ?View Solution
- 9${H_2}O,N{H_3},C{H_4},C{O_2}$ માં બંધ ખૂણાનો સાચો ક્રમ નીચેનામાંથી ક્યો હશે?View Solution
- 10$XeF_4 , XeF^-_5$ અને $SnCl_2$ના આકારો અનુક્રમે...View Solution
