$[BF_4^{-}]$માં બોરોન ની સહસંયોજકતા $(covalency)$ અને ઓકિસડેશન અવસ્થા અનુક્રમે શોધો.
JEE MAIN 2023, Medium
a
Number of covalent bond formed by Boron is \(4\)
Number of covalent bond formed by Boron is \(4\)
Oxidation number of fluorine is \(-1\),
Oxidation number of \(B +4 \times(-1)=-1\),
Thus, Oxidation number of \(B =+3\)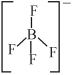
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1નીચેનામાંથી કઇ કક્ષા $\pi$ તેમજ $\delta$- બંધ બનાવી શકતી નથી?View Solution
- 2$CH _{4}, NH _{4}+$ અને $BH _{4}^{-}$ને ધ્યાનમાં લઈ નીચેનામાંથી સાચો વિકલ્પ શોધો.View Solution
- 3View Solutionનીચેનામાંથી ક્યો ઘટક અનુચુંબકીય નથી?
- 4View Solutionબહારની કક્ષકો સિવાય પરમાણુના બાકીના ભાગને ...... કહે છે.
- 5$F, Cl, Br$ અને $I$ ની વિધતઋણતા અનુક્રમે $4.0, 3.0, 2.8$ અને $2.5$ છે. તો મહત્તમ આયનીય લાક્ષણિકતા ધરાવતો હાઇડ્રોજન હેલાઇડ ક્યો થશે ?View Solution
- 6View Solutionનીચેનામાંથી કોની ધુવીયકરણ ક્ષમતા સૌથી વધુ છે ?
- 7View Solutionનીચે પૈકી શેમાં સૌથી વધારે દ્વિધ્રુવીય ચાકમાત્રા હશે?
- 8ઓર્થો-નાઇટ્રોફિનોલ એ $p-$ અને $m-$ નાઇટ્રોફિનોલ્સ કરતાં પાણીમાં ઓછું દ્રાવ્ય છે કારણ કે:View Solution
- 9View Solutionનીચેનામાંથી શેમાં બંધ ખૂણો સૌથી ઓછો હશે?
- 10કેલ્શિયમ અને ક્લોરિન સંયોજાઇ $CaCl_2$ બનાવે છે, પરંતુ $CaCl$ બનાવતા નથી. કારણ કે.....View Solution
