ભૌમિતિક સમઘટકતા દર્શાવતો આલ્કીન ............છે.
AIEEE 2009, Medium
b
The alkene that exhibits geometrical isomerism is. \(2-\)Butene may exist as cis and trans isomers. The cis-isomer has the two methyl groups on the same side and the trans-isomer has the two methyl groups on opposite sides. Due to restricted rotation around double bond it exhibits geometrical isomerism
The alkene that exhibits geometrical isomerism is. \(2-\)Butene may exist as cis and trans isomers. The cis-isomer has the two methyl groups on the same side and the trans-isomer has the two methyl groups on opposite sides. Due to restricted rotation around double bond it exhibits geometrical isomerism
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1View Solutionકયો પદાર્થ ચલરૂપકતા સ્વરૂપે અસ્તિત્વ ધરાવે છે ?
- 2View Solutionતે કિરાલ કેન્દ્ર ધરાવે છે. તો તે .....
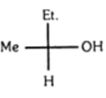
- 3બે પદાર્થો $\text{ }C{{H}_{3}}\,-\,\,C{{H}_{2}}\,-\,\,\overset{\overset{O}{\mathop{||}}\,}{\mathop{C}}\,\,\,-\,\,C{{H}_{2}}\,-\,\,C{{H}_{2}}\,-\,\,C{{H}_{3}}\,$ અને $C{{H}_{3}}\,-\,\,\overset{\overset{O}{\mathop{||}}\,}{\mathop{C}}\,\,\,-\,\,\overset{\overset{C{{H}_{3}}}{\mathop{|}}\,}{\mathop{\underset{\underset{C{{H}_{3}}}{\mathop{|}}\,}{\mathop{C}}\,}}\,\,\,-\,\,C{{H}_{3}}$ ને .......રીતે લઈ શકાય.View Solution
- 4નીચેનામાંથી કયું બંધારણએ કિરાલ કેન્દ્ર આગળ $R -$ વિન્યાસ ધરાવે છે ?View Solution
- 5View Solutionનીયો પેન્ટેનના મોનોબ્રોમો સમઘટકોની સંખ્યા ........ થશે.
- 6View Solutionનીચે આપેલા બંધારણોમાં, સૌથી વધુ દ્વિતલોકણ સાથે ક્યું એક સાંતરિત સંરુપણ (સ્ટેગર્ડ સંરૂપણ) ધરાવે છે ?
- 7View Solutionઆપેલ સંયોજન દ્વારા રચાયેલા અવકાશીયરસાયણ ની સંખ્યા કેટલી છે ?
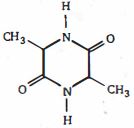
- 8View Solutionઅણુને ક્યારે કિરાલ કહી શકાય છે ?
- 9View Solutionગ્લુકોઝના શક્ય સમઘટકોની સંખ્યા .......... થશે.
- 10View Solutionનીચેનામાંથી કઈ જોડી પ્રતિબિંબી વિન્યાસ સમઘટકતા દર્શાવે છે ?
