ભૌમિતિક સમઘટકતામાં અસમાન હોય છે
AIPMT 2002, Diffcult
c
They have the same molecular formula and the same connectivity of atoms but differ in spatial arrangements of groups.
They have the same molecular formula and the same connectivity of atoms but differ in spatial arrangements of groups.
Geometrical isomerism is observed due to restricted rotation around the carbon-carbon double bond.
In geometrical isomerism there is no change in the position of the double bond or there is no change in the \(C - C\) bond length there is only a change in the spatial arrangement of the groups across the double bond.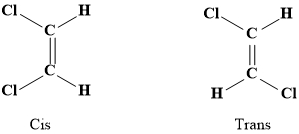
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1View Solutionકયો પદાર્થ ચલરૂપકતા દર્શાવે છે ?
- 2નીચેના સંયોજનમાં કયા નંબરના કાર્બન સાથે નવો સમૂહ જોડતાં કીરાલ સંયોજન બને ? $1_{CH_3} - 2_{CH_2}- 3_{CH_2} - 4_{CH_2} - 5_{CH_2} - 6_{CH_2} - 7_{CH_3}$View Solution
- 3નીચેનામાંથી સંયોજન માંથી $\pi$ બંધમાં ભૌમિતિક સમઘટક નહીં બતાવશે?View Solution
- 4નીચેનામાંથી સંયોજન માંથી $\pi$ બંધમાં ભૌમિતિક સમઘટક નહીં બતાવશે?View Solution
- 5$n-$ બ્યુટેનના નીચેના સંરૂપણ સમઘટકમાં તેમની વધતી પોટેન્શિયલ ઊર્જાના ક્રમમાં ગોઠવો:View Solution
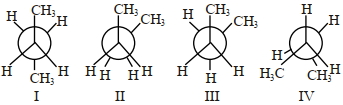
- 6$C_8H_{16}$ જે સીસ-ટ્રાન્સ ભૌમિતિક સમઘટકતાની રચના કરી શકે છે અને તેનું કિરાલ કેન્દ્ર પણ છે, જેView Solution
- 7નીચેનામાંથી કયું બંધારણએ કિરાલ કેન્દ્ર આગળ $R -$ વિન્યાસ ધરાવે છે ?View Solution
- 8View Solutionનીચેનામાંથી કયા પરમાણુ કિરાલ છે?

- 9કયો ઇથેન $-1,2-$ડાયોલ સૌથી સ્થાયી સંરુપણ છે?View Solution
- 10View Solutionબ્યુટાઈલ આલ્કોહોલ અને આઈસો બ્યુટાઈલ આલ્કોહોલમાં કયા પ્રકારની સમઘટકતા જોવા મળે છે ?
