બંધકારક -બંધકારક ઇલેક્ટ્રોકયુગ્મો વચ્ચે $90^o$ ના ખૂણાની મહત્તમ સંખ્યા ....... માં છે.
AIEEE 2004, Medium
d
(d) $dsp^2$ hybridization (Four $90^o$ angles between bond pair and bond pair)
(d) $dsp^2$ hybridization (Four $90^o$ angles between bond pair and bond pair)
$(1)$
$sp^3d$ hybridization (Six $90^o$ angle between bond pair and bond pair)
$(2)$
$sp^3d^2$ hybridization (Twelve $90^o$ angle between bond pair and bond pair)
$(3)$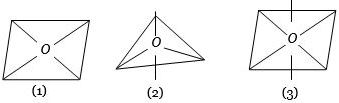
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1View Solutionનીચેના માંથી ક્યુ સંયોજન સંહસયોજ્ક છે
- 2View Solutionનીચેનામાંથી શેમાં બંધ ખૂણો સૌથી ઓછો હશે?
- 3View Solutionનીચે આપેલા અણુઓમાં કયાની પ્રકૃતિ અધ્રુવીય (non-polar) છે?
- 4View Solutionનીચેનામાંથી કઇ જોડ સમબંધારણીય છે ?
- 5View Solutionનીચેનામાંથી શેમાં આયનિક અને સહસંયોજક બંધ ધરાવે છે?
- 6View Solutionનીચેના માંથી ક્યો બંધ સૌથી વધુ સંહસયોજ્ક છે
- 7${H_2}S,N{H_3},B{F_3}$ અને $Si{H_4}$ માં બંધકોણનો સાચો ક્રમ ..........View Solution
- 8ટ્રાયગોનલ (ત્રિકોણીય) દ્રિપિરામડલ આકર ધરાવતા આણુઓ / આયન / નોની સંખ્યા___________ છે.View Solution
$\mathrm{PF}_5, \mathrm{BrF}_5, \mathrm{PCl}_5,\left[\mathrm{PtCl}_4\right]^{2-}, \mathrm{BF}_3, \mathrm{Fe}(\mathrm{CO})_5$
- 9નીચેના પરમાણુમાં કોની સૌથી નીચી $O-O$ બંધ લંબાઈ છેView Solution
- 10View Solutionનીચેનામાંથી ક્યો ધટક સામાન્ય પરિસ્થિતિમાં અસ્તિત્વ ધરાવતો નથી ?
