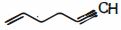
$CH_2=CH-CH_2-CH_2,-C \equiv CH$ સંયોજનમાં $C_2 -C_3$ બંધમાં નીચેના પૈકી સંકૃત કક્ષકોની કઈ જોડ સંકળાયેલી છે ?
Easy
d
\(\mathop C\limits^6 {H_2} = \mathop C\limits^5 H - \mathop C\limits^4 {H_2} - \mathop C\limits^3 {H_2} - \mathop C\limits^2 \equiv \mathop C\limits^1 H,\)
\(\mathop C\limits^6 {H_2} = \mathop C\limits^5 H - \mathop C\limits^4 {H_2} - \mathop C\limits^3 {H_2} - \mathop C\limits^2 \equiv \mathop C\limits^1 H,\)
In the given organic compound, the carbon atoms numbered as \(1,2,3,4,5,\) and \(6\) are \(s p, s p, s p^{3}, s p^{3}, s p^{2},\) and \(s p^{2}\) hybridized respectively. Thus, the pair of hybridized orbitals involved in the formation of \(C _{2}- C _{3}\) bond is \(s p-s p^{3}\)
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1નીચેના સંયોજન નું $IUPAC$ નામ લખોView Solution
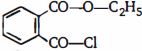
- 2નીચેના સંયોજનનું $IUPAC$ નામ શું છે?View Solution
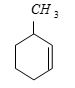
- 3$IUPAC$ નામ શું હશેView Solution
- 4$C_5H_{10}O $ અણુસૂત્રમાં કુલ કીટોનની સંખ્યા કેટલી છે ?View Solution
- 5ટર્પીંન કેમ્ફોર નું $IUPAC$ નામ શું થાય છે ?View Solution
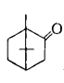
- 6પદાર્થનું $ IUPAC$ નામ જણાવો.View Solution
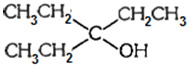
- 7$\begin{array}{*{20}{c}}View Solution
{OH\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,O} \\
{|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,||} \\
{C{H_3} - C - C{H_2} - C - C{H_3}} \\
{\,|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,} \\
{H\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}
\end{array}$ નું $IUPAC$ નામ શું હશે ? - 8$CH_2 = CH - CH_2 - NH_2 $ પદાર્થનું સામાન્ય નામ શું હશે ?View Solution
- 9પદાર્થનું ${\text{IUPAC}}\,$ નામ લખોView Solution
$\,{H_3}C\, - \,\,\mathop C\limits^{\mathop {||}\limits^O } \,\, - \,\,\mathop C\limits^{\mathop |\limits^{OH} } H\,\, - \,\,\mathop C\limits^{\mathop |\limits^{N{O_2}} } H\,\, - \,\,C{O_2}H$
- 10સાચું $IUPAC$ નામ શું હશે ?View Solution