$CHCl_3, CH_4$ અને $SF_4$ માંથી, કયા અણુઓ નિયમિત ભૂમિતિ ધરાવતા નથી?
Diffcult
b
As $CHCl _3$ has $Cl$ more electronegative than $H$ so it has not regular geometry Regular geometry of $CH _4$ is attributed to zero dipole moment $(\mu=0)$ and absence of lone pair on the carbon atom.
As $CHCl _3$ has $Cl$ more electronegative than $H$ so it has not regular geometry Regular geometry of $CH _4$ is attributed to zero dipole moment $(\mu=0)$ and absence of lone pair on the carbon atom.
$SF _4$ has lone pair on $S$ so it will increase bond angle due to repulsion hence $CHCl _3$ and $SF _4$ has not regular geometry.
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1View Solutionઅધાતુ તત્વો વચ્ચે મહત્તમ સહસંયોજકતા ધરાવતો બંધ નીચેનામાંથી શેમાં જોવા મળે છે?
- 2$MOT$ મુજબ, ${O}_{2}^{2-}$માં અયુગ્મિત ઇલેક્ટ્રોન(ઓ)ની સંખ્યા $......$ છે.View Solution
- 3નાઇટ્રોજનના અણુ ${NO}_{2}^{-}, {NO}^{+} _2$ અને ${NH}_{4}{ }^{+}$માં ભ્રમણકક્ષાના સંકરણ અનુક્રમે શું છે?View Solution
- 4View Solutionસંસ્પંદન રચનાઓ માટે નીચેનીમાંથી કઈ સ્થિતિ યોગ્ય નથી?
- 5View Solutionનીચેની જોડોમાંથી કઇ જોડ સમબંધારણીય નથી?
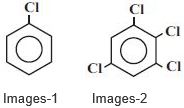
- 6$Images-1$ ની દ્વિધુવ ચાકમાત્રા $1.5 \,D$ હોય, તો $Images-2$ ની દ્વિધુવ ચાકમાત્રા .................. $\mathrm{D}$ થશે.View Solution
- 7એક દ્વિ પરમાણુક અણુમાં મુખ્ય ધરી $Z$ છે. તો ${P_x}$ અને ${P_y}$ કક્ષકોના સંપાતથી નીચેનામાંથી ક્યો બંધ બનશે?View Solution
- 8$S_2Cl_2$ની રચના કોની સાથે સમાન છેView Solution
- 9$I{F_5}$માં સંકરણનો પ્રકાર ક્યો હશે?View Solution
- 10View Solutionબંધ ખૂણાનો ઘટતો ક્રમ નીચેનામાંથી ક્યો છે?
