ચક્રીય હાઇડ્રોકાર્બન જેમાં બધા કાર્બન અને હાઇડ્રોજન પરમાણુ એક જ સમતલમાં હોય છે. બધા કાર્બન-કાર્બન બંધો સમાન લંબાઈના અને $1.54\,\mathop A\limits^o $ કરતાં ઓછા છે, પરંતુ $1.34\,\mathop A\limits^o $ કરતાં વધુ લંબાઈના હોય છે ,તો બંધ કોણ હશે?
AIPMT 1989, Medium
a
\((a)\) Cyclic hydrocarbon in which all the carbon atoms are present in the same plane is benzene. In this \(C - C\) bond length is \(1.39\,\mathop A\limits^o \) which is more than \(1.34\,\mathop A\limits^o \) but less than \(1.54\,\mathop A\limits^o \). Hence bond angle is \({120^o}\) with \(s{p^2}\) hybridization.
\((a)\) Cyclic hydrocarbon in which all the carbon atoms are present in the same plane is benzene. In this \(C - C\) bond length is \(1.39\,\mathop A\limits^o \) which is more than \(1.34\,\mathop A\limits^o \) but less than \(1.54\,\mathop A\limits^o \). Hence bond angle is \({120^o}\) with \(s{p^2}\) hybridization.
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1View Solutionનીચેના સંયોજનોની ઘનતાનો સાચો ઘટતો ક્રમ છે:
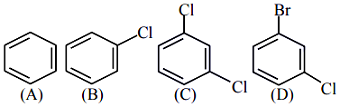
- 2$1$-બ્રોમો-$3$-ક્લોરો સાયક્લો બ્યુટેન ઉપર ઈથરમાં $2$ તુલ્યતા ધરાવતા સોડિયમ સાથે પ્રક્રિયા કરતા શું આપે છે ?View Solution
- 3View Solutionહાઇડ્રોકાર્બનના સંપૂર્ણ ઓક્સિડેશનથી હંમેશાં........... નીપજ મળે છે.
- 4View Solutionબેન્ઝિન ચક્રમાં નાઈટ્રોસમુહની હાજરીએ.....
- 5View Solutionનીયે આપેલ એક કરતાં વધારે તબક્કાવાળી પ્રક્રિયામાં બનતી નીપજ શોધો.
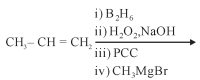
- 6$\begin{array}{*{20}{c}}View Solution
{C{H_3}\,\,\,\,\,\,\,} \\
{|\,\,\,\,\,\,\,\,\,\,\,\,\,} \\
{C{H_3} - CH - C \equiv CH}
\end{array}\xrightarrow{{excess\,HBr}}$ઉપરોક્ત પ્રકિયા માં નીપજ શું હશે ?
- 7સિસ $-2-$ બ્યુટિનમાં $Br_2$ ઉમેરતા ........... મળે છે.View Solution
- 8View Solutionઇથાઇલ કલોરાઇડમાંથી ઇથીનની બનાવટની પ્રક્રિયા...... તરીકે ઓળખાય છે.
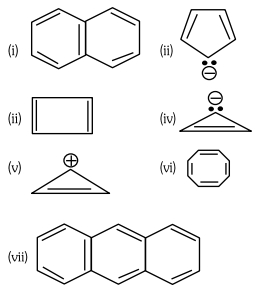
- 9નીચે આપેલા સંયોજનો ધ્યાનમાં લોView Solution
ઉપર આપેલ સંયોજનો પૈકી, સંયોજનોની સંખ્યા કે જે હ્યુકેલના નિયમનું પાલન કરે છે તે$..........$
- 10$FeCl_3$ની હાજરીમાં $Cl_2$ સાથે ટોલ્યુઇનની પ્રક્રિયા $X$ આપે છે અને પ્રકાશની હાજરીમાં પ્રક્રિયા $Y$ આપે છે આમ, $X$ અને $Y$ છે,...View Solution
