Download our appand get started for free
Similar Questions
- 1નીચેની પ્રક્રિયાઓ માટે મુખ્ય નિપજો બતાવવામાં આવે છેView Solution
${H_2}C = CH - CH = C{H_2}\xrightarrow[{0{\,^o}C}]{{HBr}}$ $\begin{array}{*{20}{c}}
{{H_2}C = CH - CH - C{H_3}} \\
{\,\,\,\,\,\,\,\,\,\,\,|} \\
{\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,Br\,\,\,\,\,\,}
\end{array}\xrightarrow{{ + 25{\,^o}C}}$ $\begin{array}{*{20}{c}}
{C{H_2}CH = CHC{H_3}} \\
{|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,} \\
{Br\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}
\end{array}$આ $......1......$ નીચા તાપમાને નિયંત્રણ અને $......2......$ ઉચ્ચ તાપમાન પર નિયંત્રણનું ઉદાહરણ પ્રદાન કરે છે.
- 2નિર્જળ $ZnCl_2$ ની હાજરીમાં $2-$ મિથાઇલ પ્રોપિનને એસિટાઇલ ક્લોરાઇડ સાથે ગરમ કરતા મળતી નીપજ .....View Solution
- 3પ્રક્રિયામાં $C{H_3} - C \equiv C - C{H_3}\xrightarrow[{(ii)\,{H_2}O/Zn}]{{(i)\,X}}\begin{array}{*{20}{c}}View Solution
{C{H_3} - C - C - C{H_3}} \\
{||\,\,\,\,\,\,\,\,\,\,||} \\
{O\,\,\,\,\,\,\,O}
\end{array};$ $X$ એ શું છે. - 4કાલ્પનિક$1, 3,5-$ સાયકલોહેકઝેટેરિન ની સાથે બેન્ઝિનના હાઇડ્રોજનને સરખામણી કરતી વખતે, બેન્ઝિન .................... સાયક્લોહેક્સેટ્રેન કરતાં.View Solution
- 5View Solutionનીચેના પ્રક્રિયા દરમિયાન કેટલા સંક્રાતિ અવસ્થા અને મધ્યસ્થીની રચના થશે?
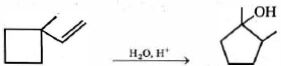
- 6$C_2H_6, C_2H_4$ અને $C_2H_2$ નું મિશ્રણ કોપર $(I)$ક્લોરાઈડ ના આલ્કલાઇન સોલ્યુશન દ્વારા પરપોટા વુલ્ફની બોટલમાં સમાયેલ છે. બહાર આવતા ગેસ કયા છે ?View Solution
- 7Conc $ H_2SO_4 $ દ્વારા નિર્જલીકરણ ના દર $(i), (ii)$ and $(iii)$ ની સરખામણીકરો.View Solution
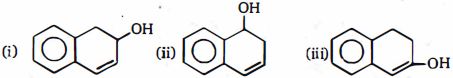
- 8$H - C\, \equiv CH\,\xrightarrow[{(ii)\,C{H_3}C{H_2}Br}]{{(i)\,NaN{H_2}\,/\,liq.\,N{H_3}}}\,X$View Solution
$\xrightarrow[{(ii)\,C{H_3}C{H_2}Br}]{{(i)\,NaN{H_2}\,/\,liq.\,N{H_3}}}\,Y$
પ્રક્રિયામાં $X$ અને $Y$ ....... છે.
- 9View Solutionનીચેના પૈકી ક્યુ સંયોજન ઇલેક્ટ્રોન અનુરાગી બ્રોમિનેશનમાં બેન્ઝિન કરતા ધીમી પ્રક્યિા કરે છે ?
- 10પ્રકિયા ની નીપજ $(B)$ શું હશે ?View Solution
$\begin{matrix}
CH-C{{O}_{2}}H \\
\,||\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\, \\
CH-C{{O}_{2}}H \\
\end{matrix}\xrightarrow[(two\,mole)]{NaOH}(A)\xrightarrow{electrolysis}(B);$
