ડાયબોરેનમાં, $H - B - H$ બંધ ખૂણો ${120^o}$ છે. બોરોનનું વર્ણસંકરણની શક્યતા ....... છે.
AIPMT 1999, Medium
c
Diborane, \(B _2 H _6\) has two electrons each, three centred bonds. Each Boron atom \((B)\) is linked with four hydrogen atoms. This makes tetrahedral geometry. Hence, each Boron atom is \(sp ^3\) hybradised.
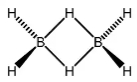
Diborane, \(B _2 H _6\) has two electrons each, three centred bonds. Each Boron atom \((B)\) is linked with four hydrogen atoms. This makes tetrahedral geometry. Hence, each Boron atom is \(sp ^3\) hybradised.

Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1$C{O_2},C{H_3}CO{O^ - },$ $CO,C{O_3}^{2 - },$ $HCHO$ આપેલ સંયોજનોમાંથી કેમાં કાર્બન-ઓક્સિજન બંધ નિર્બળ છેView Solution
- 2નીચે આપેલી જોડીઓમાંથી કઈ એકમાં મધ્યસ્થ પરમાણુઓ $\mathrm{sp}^2$ સંકરણ પ્રદર્શિત કરે છે?View Solution
- 3કયા ઘટકનો આકાર $NH_3$ જેવો છે?View Solution
- 4View Solutionનીચેનામાંથી ક્યા તત્વની સંયોજકતા બદલાયા વગરની રહે છે?
- 5View Solutionકયો પરમાણુ પિરામિડ આકારનો છે ?
- 6ખોટુ જોડકૂ શોધોView Solution
ઇલેક્ટ્રોન ભૂમિતિ $-$ સંબંધિત ઇલેક્ટ્રોન ભૂમિતિથી શક્ય પરમાણુ આકાર
- 7દ્વિધુર્વીય ચાકમાત્રા વધારવા માટે નીચેના સંયોજનો ગોઠવો.View Solution
(I) ટોલ્યુઇન
(II) $m - $ ડાયક્લોરોબેંઝિન
(III) $o -$ ડાયક્લોરોબેંઝિન
(IV)$p - $ ડાયક્લોરોબેંઝિન
- 8View Solutionનીચેના માંથી ક્યા સંયોજનમાં સૌથી વધુ દ્વિધ્રુવ ચાકમાત્રા હશે?
- 9$MX_3$ અણુની દ્વિધુવ ચાકમાત્રા શૂન્ય હોય, તો સિગ્મા બંધનમાં $M \,(atomic\, number < 21)$ દ્વારા ઉપયોગમાં લેવાતી કક્ષકો ....View Solution
- 10View Solutionનીચેનામાંથી શામાં સહસંયોજક બંધ છે?
