Here, \(R=31.6\,\,ohm\)
\(\therefore \,\,C\, = \,\frac{1}{R}\, = \,\frac{1}{{31.6}}\,oh{m^{ - 1}}\,\, = \,0.0316\,oh{m^{ - 1}}\)
Specific conductance
\(=\) conductance \(\times \) cell constant
\( = \,0.0316\,oh{m^{ - 1}}\,\, \times \,\,0.367\,c{m^{ - 1}}\)
\( = \,0.0116\,\,oh{m^{ - 1}}c{m^{ - 1}}\)
Now, molar concentration \(=\,0.5\,M\) (given)
\(=\,0.5\times 10^{-3}\,mole\,cm^{-3}\)
\(\therefore \) Molar conductance \(=\,\frac {k}{molar \,conc.}\)
\(=\,\frac {0.0116}{0.5\times 10^{-3}}\)
\(=\,23.2\,S\,cm^2\,mol^{-1}\)
Download our appand get started for free
Similar Questions
- 1નીચેની પ્રક્રિયા માટે કોષનો $ emf$ આપેલ છે. $Zn (s) + Ni^{2+}_{(aq)}\, (a = 0.1) \rightarrow Zn^{2+}_{(aq)}\, (a = 1.0) + Ni\,\, 298\,K$. એ $0.5105\, V$ જોવા મળે છે કોષમાં પ્રમાણિત $e$ કેટલા. ................ $\mathrm{V}$ છે?View Solution
- 2કોપર ધાતુ સાથે સિલ્વર આયનના રિડકશન માટે વપરાતા કોષનો પ્રમાણિત કોષ પોટેન્શિયલ $25\,^{o} C$ તાપમાને $0.46\,V$ હોય, તો ગિબ્સ ઊર્જા $(\Delta G^o) $ નું મૂલ્ય કેટલા થાય? $(F= 96500\,C\,mol^{-1})$View Solution
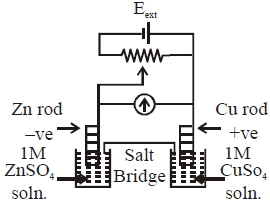
- 3$1.1\,V.$ ના $Zn\,|\,Z{n^{2 + }}\,(1\,M)\,\,||\,\,C{u^{2 + }}\,(1\,M)\,|\,Cu,$ કોષને એક ચલિત, વિરુદ્ધ બાહ્ય પોટેન્શિયલ $(E_{ext})$ લાગુ પાડવામાં આવ્યો છે. જયારે $E_{ext} < 1.1\,V$ અને $E_{ext} > 1.1\,V,$ હોય ત્યારે ઇલેક્ટ્રોન, વહત અનુક્રમે રૂ થાય છે.View Solution
- 4View Solutionગેલ્વેનાઇઝિંગ એ નીચેનામાંથી કોનુ અસ્તર હોય છે ?
- 5$E _{ Cu ^{2+} \mid Cu }^{\circ}=+0.34 V$View Solution
$E _{ Zn ^{2}+\mid Zn }^{ o }=-0.76 V$
ઉપરોક્ત કોષ માટે નીચેના વિકલ્પોમાંથી ખોટા વિધાનની ઓળખ આપો
- 6ઘડિયાળમાં વપરાતો button cell નીચે મુજબ કાર્ય કરે છે.View Solution
$Zn_{(s)} + Ag_2O_{(s)} + H_2O_{(l)} \rightleftharpoons $$2Ag_{(s)} + Zn^{2+}_{(aq)}+ 2OH^-_{(aq)}$
જો અર્ધકોષ પોટેન્શિયલ
$Zn^{2+}_{(aq)} + 2e^- \rightarrow Zn_{(s)}\,;\,\, E^o = - 0.76\, V$
$Ag_2O_{(s)} + H_2O_{(l)} + 2e^- \rightarrow 2Ag_{(s)} + 2OH^-_{(aq)}\,,$$E^o = 0.34\, V$
હોય, તો કોષ-પોટેન્શિયલ ......... $V$ થશે.
- 7$1\,A$ વિદ્યુત પ્રવાહ $1$ સેકન્ડ માટે પસાર કરતાં નિક્ષેપન પામતા પદાર્થનું પ્રમાણ .....જેટલું હોય છે.View Solution
- 8View Solutionકઈ પ્રક્રિયાના સમીકરણ દ્વારા ઝીંક નો પ્રમાણિત રીડક્શન પોટેશિયલ દર્શાવી શકાય?
- 9$298\,K$ તાપમાને કોષ નું $e.m.f.$ $Zn|Z{n^{2 + }}(0.01\,M)||F{e^{2 + }}(0.001\,M)|Fe$ $0.2905 $ છે પછી કોષની પ્રક્રિયા માટે સંતુલનનું મૂલ્ય કયું છેView Solution
- 10બે ઇલેક્ટ્રોનનો ફેરફાર ધરાવતી કોષપ્રક્રિયા માટે, ${25\,^o}C$ પર કોષનો પ્રમાણિત $e.m.f\,\,0.295\,V $ મળે છે. તો ${25\,^o}C$ પણ પ્રક્રિયાનો સંતુલન અચળાંક ગણો.View Solution
