Since the compound undergoing combustion is an alkane. Hence the combustion reaction can be written as
\(\underset{5L}{\mathop{{{C}_{n}}{{H}_{2n+2}}}}\,+\underset{25L}{\mathop{\left( \frac{3n+1}{2} \right){{O}_{2}}}}\,\to \) \(nC{{O}_{2}}+(n+1){{H}_{2}}O\)
since volumes are measured at constant \(T\) & \(P\). Hence according to Avogadro's law
Volume \(\propto \) mole
\(\therefore \,{{n}_{alkane}}=\left( \frac{2}{3n+1} \right)\times {{n}_{{{O}_{2}}}}\)
\(5 = \frac{2}{{3n + 1}} \times 25\)
\(\therefore \,n = 3\)
Hence alkane is propane \((C_3H_8)\)
Download our appand get started for free
Similar Questions
- 1View Solutionક્યા પ્રક્રિયકોની શ્રેણી દ્વારા એસિટિલિનનું ઉચ્ચ આલ્કાઇનમાં રૂપાંતર કરી શકાય છે ?
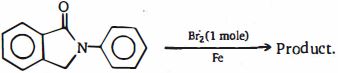
- 2View Solutionપ્રકિયા ની મુખ્ય નીપજ કઈ છે ?
- 3View Solutionનીચે આપેલ પ્રક્રિયામાં બનતી નીપજ શોધો.
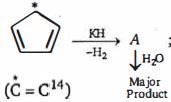
- 4એક ક્લોરો સંયોજન $"A"$View Solution
$(i)$ ઓઝોનોલિસિસ પર આલ્ડિહાઈડ્સ બનાવે છે, ત્યારબાદ જલીયકરણ થાય છે.
$(ii)$ જ્યારે ${A}$નું $1.53\, {~g}$ સંપૂર્ણપણે બાષ્પીભવન થાય છે,$STP$ પર $448\, {~mL}$ બાષ્પ આપે છે.
સંયોજન $A$ના પરમાણુમાં કાર્બન અણુઓની સંખ્યા ...... છે.
- 5સંયોજન $(X)$એ ..........View Solution
સંયોજન $(X)$ $\xrightarrow[{Pt}]{{5{H_2}}}$
સંયોજન $(X)$ $\xrightarrow{{AgN{O_3}}}$ અવક્ષેપિત
સંયોજન $(X)$ $\xrightarrow[{M{e_2}S}]{{{O_3}}}$ $\begin{array}{*{20}{c}}
{\,\,\,\,\,\,\,O\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,O\,\,\,\,\,\,\,\,\,\,O\,\,\,\,} \\
{\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,||\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,||\,\,\,\,\,\,\,\,\,\,||\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,} \\
{H - C - C{H_2} - C{H_2} - C - C - H}
\end{array}$ $\begin{array}{*{20}{c}}
{\,\,O\,\,\,\,\,\,O\,\,\,\,\,\,\,\,\,\,} \\
{||\,\,\,\,\,\,\,\,||\,\,\,\,\,\,\,\,\,} \\
{H - C - C - O - H}
\end{array}$ $ + \begin{array}{*{20}{c}}
{O\,\,\,\,\,\,\,} \\
{||\,\,\,\,\,\,\,\,} \\
{H - C - O - H}
\end{array}$ $ + \begin{array}{*{20}{c}}
{CHO} \\
{|\,\,\,\,\,\,\,\,} \\
{CHO}
\end{array}$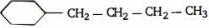
- 6ઇથાઇલ બેન્ઝિનના $KMnO_4$ વડે ઓક્સિડેશનથી કઇ નીપજ મળે છે ?View Solution
- 7સંયોજન $(A)$ શું હશે ?View Solution
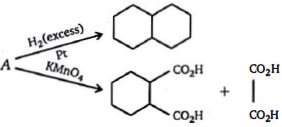
- 8નીચેની પ્રક્રિયાની મુખ્ય નીપજ કઈ છે ?View Solution
$\begin{array}{*{20}{c}}
{C{H_3}\,\,\,\,\,\,\,} \\
{|\,\,\,\,\,\,\,\,\,\,\,\,\,} \\
{{H_3}C - C - CH = C{H_2}} \\
{|\,\,\,\,\,\,\,\,\,\,\,\,\,} \\
{C{H_3}\,\,\,\,\,\,\,\,\,}
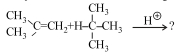
\end{array}$ $\xrightarrow{{{H_2}O/{H^ \oplus }}}{\mkern 1mu} \mathop A\limits_{Major\,product} \, + \,\mathop B\limits_{Minor\,product} $ - 9નીપજ $(C)$ શું હશે ?View Solution
(figure) $\xrightarrow[CC{{l}_{4}}]{B{{r}_{2}}}(A)\xrightarrow[(ii)\,NaN{{H}_{2}}]{(i)\,alc.\,KOH}(B)\xrightarrow[(ii)\,C{{H}_{3}}-Cl]{(i)\,NaN{{H}_{2}}}(C)$
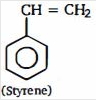
- 10View Solutionનીચે આપેલી પ્રકિયા માં મુખ્ય નીપજ બને છે કઈ છે ?