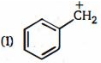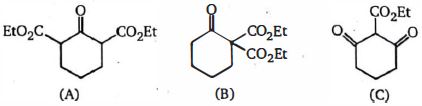
The greater \(I\) effect is favorable or greater acidic strength. The \(-I\) effect of aldehyde group is greater than the ester group. Due to this \(C\) will be most acidic. In \(A\) and \(B , B\) will be less acidic as it has \(-I\) effect sort of localized due to ester group being on a single carbon. In \(A\) it is more delocalized is not effective on more by adjacent carbons.
Hence, the required order is- \(B\, <\, A\, <\, C\).
Download our appand get started for free
Similar Questions
- 1View Solutionઆપેલ કઈ જોડીઓમાં, જેમાં જોડીનું બીજું સંયોજન પ્રથમ કરતા વધુ એસિડિક છે
- 2નીચેના માટે એસિડિક ક્ષમતાનો સાચો ક્રમ લખોView Solution
$(I)$ $ CH_3 - NO_2$
$(II)$ $NO_2 - CH_2 - NO_2$
$(III)$ $ CH_3 - CH_2 - NO_2 $
$(IV)$ $\begin{array}{*{20}{c}}
{N{O_2} - CH - N{O_2}} \\
{|\,\,\,\,\,\,} \\
{\,N{O_2}}
\end{array}$ - 3નીચેના ત્રણ કાર્બોનિયમ આયનોની સ્થિરતાનો યોગ્ય ક્રમ કયો છે?View Solution
$(I)$ $C{H_2} = CH\mathop C\limits^ + HC{H_3}$
$\begin{array}{*{20}{c}}
{{\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} \,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,C{H_3}} \\
{\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,|\,\,} \\
{(II)\,\,\,\,\,\,\,\,\,\,C{H_2} = C - \mathop {{\text{ }}C}\limits^ + {H_2}}
\end{array}$$(III)$ $C{H_3}CH = CH\mathop C\limits^ + {H_2}$
- 4$CH_3CH_2Cl + KOH(aq.) \rightarrow CH_3CH_2 - OH + KCl $ ઉપરની પ્રક્રિયા કઈ છે ?View Solution
- 5View Solutionનીચેનામાંથી કોણ સૌથી ઓછો એસિડિક છે ?
- 6View Solutionનીપજની સ્થિરતાની સમજના આધારે, જ્યારે નીચેનો ડાયએનાયન એસિડના સમકક્ષ સાથે પ્રક્રિયા આપે ત્યારે રચાયેલી નીપજ ની આગાહી કરો
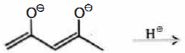
- 7આપેલ સંયોજનમાં $\alpha$ -હાઇડ્રોજનની કુલ સંખ્યા કેટલી છેView Solution
- 8એસિડ પ્રબળતા નો ઘટાડવાનો ક્રમ કયો છે ?View Solution
$\mathop {Ph - OH}\limits_{(A)} ,\,\,\,\,\mathop {Ph - C{H_2} - OH}\limits_{(B)} ,\,\,\,\,\mathop {Ph - C{O_2}H}\limits_{(C)} ,\,\,\,\mathop {Ph - C{H_2}-\mathop N\limits^ \oplus {H_3}}\limits_{(D)} $
- 9નીચેનામાંથી ક્યો આલ્કાઇલ હેલાઇS $E_2$ ક્રિયાવિધિ પ્રત્યે સૌથી સક્રિય છે ?View Solution
- 10નીચે લખેલા ક્યાંક $I, II$ અને $III$ ની સંબંધિત સક્ષમતાના યોગ્ય ક્રમમાં નીચેનામાંથી કયા વિકલ્પો છે (સૌથી સ્થાયી પહેલા)?View Solution
$(II)\,\,\,{{H}_{2}}C=CH-C{{H}_{2}}-\overset{+}{\mathop{C}}\,H-C{{H}_{3}}$
$(III)\,\,\,\begin{matrix}
\,\,\,\,\,C{{H}_{3}}\, \\
|\, \\
{{H}_{3}}C-C-\overset{+}{\mathop{C}}\,{{H}_{2}} \\
|\, \\
\,\,\,C{{H}_{3}} \\
\end{matrix}$