${H_2}O$ દ્વિધ્રુવીય છે, જયારે $BeF_2$ નથી. કારણ કે .............
AIPMT 2004,AIPMT 1989, Medium
b
(b)The overall value of the dipole moment of a polar molecule depends on its geometry and shape i.e., vectorial addition of dipole moment of the constituent bonds water has angular structure with bond angle \(105°\) as it has dipole moment.
(b)The overall value of the dipole moment of a polar molecule depends on its geometry and shape i.e., vectorial addition of dipole moment of the constituent bonds water has angular structure with bond angle \(105°\) as it has dipole moment.
However \(Be{F_2}\) is a linear molecule since dipole moment summation of all the bonds present in the molecule cancel each other. 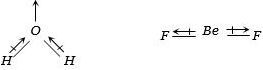
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1View Solutionનીચેનામાંથી કયા આયન / પરમાણુમાં સમાન સંખ્યામાં બંધકારક ઇલેક્ટ્રોનયુગ્મ અને અબંધકારક ઇલેક્ટ્રોનયુગ્મ નથી?
- 2View Solutionનીચેનામાંથી ક્યા સંયોજનમાં ઇલેક્ટ્રોનની ઘટ છે?
- 3View Solutionનીચેનામાંથી સૌથી વધુ સ્નિગ્ધ ક્યું હશે?
- 4View Solutionટોલ્યુઇનમાં સિગ્મા અને પાઇ બંધોની સંખ્યા કેટલી છે?
- 5View Solutionનીચેનામાંથી ક્યો બંધ સૌથી નબળો છે?
- 6View Solutionબે પરમાણુઓ વચ્ચે સહસંયોજક બંધ નીચેના માંથી કોના દ્વારા બને છે
- 7આપેલ હાઇડ્રાઈડ $Ca{H_2},N{H_3},NaH$ અને ${B_2}{H_6}$ માંથી ક્યુ હાઇડ્રાઈડ સહસંયોજક છેView Solution
- 8View Solutionનીચેનામાંથી સંયોજનમાં દ્વિધ્રુવીય ચાકમાત્રા લગભગ ક્લોરોબેન્ઝિન જેટલું જ સમાન છે?
- 9In $HCHO,\,\,'C'$નું સંકરણ શું હશે ?View Solution
- 10નીચે આપેલ સ્પીસીઝો માંથી મધ્યસ્થ પરમાણું પર ઈલેકટ્રોનો અબંધકાર યુગ્મો ની મહત્તમ સંખ્યા $..........$ $ClO _3^{-}, XeF _4, SF _4,I _3^-$View Solution
