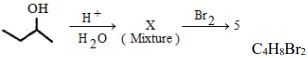Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1View Solution......... બંધ સૌથી વધુ એસિડિક છે.
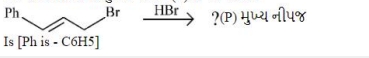
- 2આપેલી પ્રક્રિયાની મુખ્ય નીપજ $(P)$ નીપજ શોધો.View Solution
- 3નીચેનામાંથી કઇ પ્રક્રિયા $2,2$-ડાયબ્રોમોપ્રોપેન નીપજ તરીકે આપશે ?View Solution
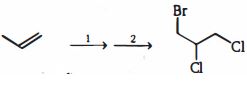
- 4View Solutionપ્રક્રિયાઓની કઈ શ્રેણી નીચેના પરિવર્તનને પ્રાપ્ત કરશે?
- 5$C{H_3} - \mathop {CH}\limits^{\mathop |\limits^{C{H_3}} } \,\, - \,\,C{H_2} - C{H_3}\,\,\xrightarrow{{C{l_2}/hv}}\,\,N$( સમઘટકોની સંખ્યા) $ \xrightarrow{{Fractional\,\,\,{\text{distillation}}}}\,\,(F)\,,\,\,(N)$ અને $\,(F)\,$ એ .............View Solution
- 6View Solutionકયુ સંયોજન વિસ્ફોટક તરીકે વપરાય છે ?
- 7View Solutionપદાર્થ બેયરની કસોટી આપે છે પણ જ્યારે તેને એમોનિયેકલ સિલ્વર નાઈટ્રેટ દ્રાવણ સાથે સફેદ અવક્ષેપ આપતા નથી ?
- 8View Solutionબતાવેલ તમામ હાઇડ્રોકાર્બન ખૂબ નબળા એસિડ્સ છે. એક જોકે અન્ય કરતા વધુ એસિડિક છે. કયું એક સૌથી મજબૂત એસિડ છે
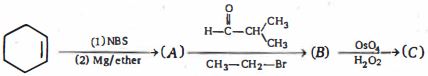
- 9નીપજ $(C)$ શું હશે ?View Solution
- 10પદાર્થનું અણુસૂત્ર છે તો $X$ પદાર્થની સંખ્યા કેટલી થાય ?View Solution