હીરા માં ક્યાં પ્રકારનો બંધ જોવા મળે છે
Easy
a
Diamond is organised in a giant lattice structure with strong covalent bonds between carbon atoms. Each carbon atom forms \(4\) bonds. Therefore it has a rigid structure, and cannot conduct electricity due to the lack of free electrons.
Diamond is organised in a giant lattice structure with strong covalent bonds between carbon atoms. Each carbon atom forms \(4\) bonds. Therefore it has a rigid structure, and cannot conduct electricity due to the lack of free electrons.
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1નીચેના સંયોજનમાં કયા કેન્દ્રિય અણુ $(^*)$ના સંકરણમાં ફેરફાર થતો નથી?View Solution
- 2સાચું જોડકું શોધોView Solution
વિધાન $-$ આકાર $-$ ઉદાહરણ
- 3પાણી માં સહસંયોજક બંધ $O-H$ ની બંધ ઉર્જા શું હશે?View Solution
- 4View Solutionનીચેનામાંથી કોની દ્વિધ્રુવીય ચાકમાત્રા શૂન્ય છે?
- 5સહસંયોજ્ક સંયોજન $HCl$ આયનિક ગુણ ધરાવે છે, જેમાંView Solution
- 6View Solutionનીચેના ઘટકોમાંથી પ્રતિચુંબકીય અણુ ક્યો છે ?
- 7$\mathrm{CO}$ અને $\mathrm{NO}^{+}$ ના બંધક્રમાંકનો સરવાળો ___________છે.View Solution
- 8View Solutionનીચેના સંયોજનમાં હાજર આણ્વિય આકર્ષણ બળનો/ના પ્રકાર કયો/કયા છે?
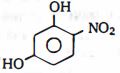
- 9ડાયઇથાઇલ ઇથર $C_2H_5OC_2H_5$ના પરમાણુમાં કેટલા સિગ્મા બંધ છે?View Solution
- 10View Solutionનીચેનામાંથી કયું આકર્ષણ સૌથી પ્રબળ છે?
