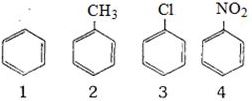
ઇલેક્ટ્રોનઅનુરાગી પ્રક્રિયક તરફની પ્રતિક્રિયાશીલતામાં ઘટાડો થવાનો ક્રમ, નીચે મુજબ હશે
$(i)$ બેન્ઝિન $(ii)$ ટોલ્યુઇન
$(iii)$ ક્લોરોબેન્ઝિન $(iv)$ ફિનોલ
AIPMT 2007, Medium
c
Benzene having any activating group i.e, oH, Retc, undergoes electrophilic substitution very easily as compared to benzene itself. Thus toluene \(\left(\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CH}_{3}\right),\) phenol \(\left(\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{O} \mathrm{H}\right)\) undergo electrophilic substitution very readily than benzene chlorine with \(+\mathrm{E}\) and \(+\mathrm{M}\) effect deactivating the ring due to strong \(-I\) effect. So, it is difficult to carry out the substitution in chlorobenzene than in benzene, so correct order is
Benzene having any activating group i.e, oH, Retc, undergoes electrophilic substitution very easily as compared to benzene itself. Thus toluene \(\left(\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CH}_{3}\right),\) phenol \(\left(\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{O} \mathrm{H}\right)\) undergo electrophilic substitution very readily than benzene chlorine with \(+\mathrm{E}\) and \(+\mathrm{M}\) effect deactivating the ring due to strong \(-I\) effect. So, it is difficult to carry out the substitution in chlorobenzene than in benzene, so correct order is
Phenol \(>\) Toluene \(>\) Benzene \(>\) Chlorobenzene
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1View Solutionનીચેની પ્રક્રિયામાં ભાગ લેતા ઈલેકટ્રોનુરાગી કયા છે ?
- 2એનીલીન $(I)$, બેન્ઝીન $(II)$ નાઈટ્રોબેન્ઝિન $(III)$ પદાર્થોના ઈલેકટ્રોન અનુરાગી વિસ્થાપનનો સાચો ક્રમ કયો છે ?View Solution
- 3View Solutionઉપરોક્ત સંયોજનોના ઉષ્મા ના દહન નો યોગ્ય ક્રમ કયો છે ?
- 4View Solutionનીચેનામાંથી કયો કાર્બન આયન સૌથી ઓછો સ્થિર છે ?
- 5ક્લોરોબેન્ઝિનની ડાયપોલ મોમેન્ટ $\mu $ છે તો ની ડાયપોલ મોમેન્ટ કેટલી થાય છે ?View Solution
- 6View Solutionનીચેના પૈકી સૌથી ઓછી દ્વિધ્રુવ ચાકમાત્રા ધરાવતો અણુ જણાવો.
- 7$2-$બ્રોમો પેન્ટનની ડિહાઈડ્રોહેલોજીનેશન પ્રક્રિયામાં મુખ્ય નીપજ પેન્ટ$-2-$ઈન બને છે. આ બનતી નિપજ આધારિત છે તે $....\,.$View Solution
- 8View Solutionકેન્દ્રઅનુરાગી વિસ્થાપન થવા માટે નીચેના પૈકી ક્યુ સૌથી સાચુ ઇલેક્ટ્રોનીય સ્થાનાંતર છે ?
- 9મુક્ત મૂલકના કયા બંધારણમાં મહત્તમ સંસ્પદન જોવા મળશે? $ [[\phi = C_6H_5]$View Solution
- 10View Solutionનીચેના પદાર્થોમાં ઈલેકટ્રોનઅનુરાગી વિસ્થાપન પ્રક્રિયામાં પ્રક્રિયાશીલતાનો સાચો ક્રમ કયો છે ?