જો $AB _{4}$ એ ધ્રુવીય અણુ છે તો $AB _{4}$ ની શક્ય ભૂમિતિ શું થશે ?
JEE MAIN 2020, Medium
a
$(1)$ If $AB _{4}$ molecule is a square pyramidal then it has one lone pair and their structure should be and it should be polar because dipole moment of lone pair of $'A'$ never be cancelled by others.
$(1)$ If $AB _{4}$ molecule is a square pyramidal then it has one lone pair and their structure should be and it should be polar because dipole moment of lone pair of $'A'$ never be cancelled by others.
$(2)$ If $AB _{4}$ molecule is a tetrahedral then it has no lone pair and their structure should be and it should be non polar due to perfect symmetry.
$(3)$ If $AB _{4}$, molecule is a square planar then it should be non polar because vector sum of dipole moment is zero.
$(4)$ If $AB _{4}$ molecule is a rectangular planar then it should be non polar because vector sum of dipole moment is zero.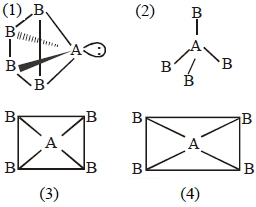
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1View Solutionનીચેનામાંથી ક્યો ઓક્સાઇડની અપેક્ષા દર્શાવે છે તે પેરામેગ્નેટિક (paramagnetic) વર્તણૂક દર્શાવે છે
- 2$S{F_4},\,C{F_4}$ તથા $Xe{F_4}$ માં આણ્વિય આકાર ........ છે.View Solution
- 3View Solutionસમાન બંધક્રમાંક ધરાવતી ઘટકોની જોડ .....
- 4$BC{l_3}$ અણુ સ્તરીય છે તેનું કારણ આ અણુમાં બોરોન નું ક્યા પ્રકારનું સંકરણ થયું હશે?View Solution
- 5View Solutionપાણી એ પ્રવાહી છે કારણ કે
- 6View Solutionઓક્સિજનની ઘટકોની જોડી અને તેના ચુંબકીય વર્તન નીચે નોંધવામાં આવે છે. નીચેનામાંથી કયો વિકલ્પ સાચા વર્ણન રજૂ કરે છે?
- 7View Solutionનીચેનામાંથી ક્યા હાઇડ્રાઇડનું ઉત્કનબિંદુ સૌથી ઓછું હશે?
- 8$XeO_4$ અણુ .. . . . ધરાવતો ચતુષ્ફલકીય અણુ છે .View Solution
- 9$ICl$ અને $Br_2$નું પરમાણુ કદ લગભગ સમાન છે, પરંતુ $b .p.$ જો $ICl$ નું $Br_2$ કરતા આશરે $40\,^oC$ વધારે છે. તે કારણ છેView Solution
- 10$p - p$ કક્ષકોના બાજુએથી ઓવરલેપ થવાથી નીચેનામાંથી ક્યો બંધ બનશે?View Solution
