જ્યારે $2-$બ્યુટીન $H_2/$લિંડલર ઉદીપક સાથે પ્રક્રિયા કરવામાં આવે છે,ત્યારે સંયોજન $X$ મુખ્ય નીપજ તરીકે મળે છે અને જ્યારે તેની $Na/liq.$ $NH_3$ સાથે પ્રક્રિયા કરવામાં આવે છે ત્યારે તે $Y$ મુખ્ય નીપજ ઉત્પન્ન કરે છે. નીચેનામાંથી કયું વિધાન સાચું છે?
JEE MAIN 2018, Advanced
d
When \(2-\) butyne is treated with \(H_2/\) Lindlar's catalyst, compound \(X\) (cis \(-2-\) butene) is produced as the major product; and when treated with \(Na/liq\,NH_3\) it produces \(Y\) (trans \(-2-\) butene) as the major product. Cisisomer \((X)\) will have higher dipole moment and higher boiling point than trans \((Y)\).
When \(2-\) butyne is treated with \(H_2/\) Lindlar's catalyst, compound \(X\) (cis \(-2-\) butene) is produced as the major product; and when treated with \(Na/liq\,NH_3\) it produces \(Y\) (trans \(-2-\) butene) as the major product. Cisisomer \((X)\) will have higher dipole moment and higher boiling point than trans \((Y)\).
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1ઉપરની પ્રક્રિયામાં $X$ શોધો:View Solution
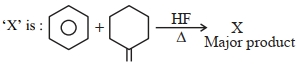
- 2સિસ- $2$ -બ્યુટીન $\xrightarrow[{Peroxide}]{{HBr}}$ નીપજ ;આપેલી પ્રકિયા ની નીપજ શું હશે ?View Solution
- 3હાઈડ્રોબોરેશન -ઑક્સિડેશન પ્રક્રિયામાં $1$-પેન્ટીન ના $2\, mole$ સાથે સંપૂર્ણ પ્રક્રિયા આપવા માટે $BH_3$ ના કેટલા મોલની જરૂર પડે છે ?View Solution
- 4ટ્રાન્સ - સાયકલોહેકઝેન $1,2$ -ડાયોલ એ સાયકલોહેકઝીન ની કોની સાથે ની પ્રકિયા થી મેળવી શકાય છે ?View Solution
- 5$1$ મોલ બેન્ઝિનનું ઓઝોનોલિસિસ ............. આપે છે.View Solution
- 6View Solutionઆલ્કેન (સાયકલોઆલ્કેન નથી ) એ કાર્બનિક અણુઓને ઈનાસ્યોમેરિક સ્વરૂપમાં અસ્તિત્વમાં રહે તે પહેલાં કેટલા કાર્બન અણુઓની જરૂર છે?
- 7નીચેની આપેલી પ્રક્રિયાઓના ક્રમમાં મુખ્ય નીપજ $[B]$ શું હશે ?View Solution
$\begin{array}{*{20}{c}}
{C{H_3} - C = CH - C{H_2}C{H_3}} \\
{|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,} \\
{CH{{(C{H_3})}_2}\,\,\,\,\,\,\,\,\,\,}
\end{array}\xrightarrow[{(ii)\,{H_2}{O_2},O{H^ - }}]{{(i)\,{B_2}{H_6}}}[A]$$\xrightarrow[\Delta ]{{dil.\,{H_2}S{O_4}}}[B]$ - 8નીચેની પ્રક્રિયાઓના ક્રમમાં એક જ સમતલમાં $'C$'માં અણુઓની મહત્તમ સંખ્યા હાજર છેView Solution
$A \xrightarrow[ { Cu\; tube }]{\text { Redhot }}\mathrm{B} \xrightarrow[ Anhydrous AlCl_3]{\mathrm{CH}_{3} \mathrm{Cl}(1 \mathrm{eq}} \mathrm{C}$
($A$ એ સૌથી ઓછું પરમાણ્વીય વજન ધરાવતું આલ્કાઇન છે)
- 9આ પ્રકિયા ની $(A)$ નીપજ શું હશે ?View Solution
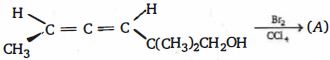
- 10View Solutionનીચા કાર્બન નંબરના હાઇડ્રોકાર્બન તૈયાર કરવાની સૌથી મહત્વપૂર્ણ પદ્ધતિ કઈ છે
