જ્યારે $Cl_2$ વાયુ ગરમ અને સાંદ્ર સોડિયમ હાઇડ્રોક્સાઇડના દ્રાવણ સાથે પ્રક્રિયા કરે ત્યારે કલોરિનનો ઓક્સિડેશન આંક ............ થી બદલાય છે.
AIPMT 2012, Medium
b
The reaction of chlorine gas with hot and concentrated sodium hydroxide solution is
The reaction of chlorine gas with hot and concentrated sodium hydroxide solution is
\(3 \mathrm{Cl}_{2}+6 \mathrm{NaOH} \rightarrow \quad \mathrm{NaClO}_{3}+5 \mathrm{NaCl}+3 \mathrm{H}_{2} \mathrm{O}\)
Oxidation number of \(\mathrm{Cl}\) is 0 in \(\mathrm{Cl}_{2},-1\) in \(\mathrm{NaCl}\) and \(+5\) in \(\mathrm{NaClO}_{3}\)
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1આપેલ પ્રક્રિયા માટે $A, B, C, D$ પસંદ કરો.View Solution
${N_2} + 3{H_2} \longrightarrow NH_3$
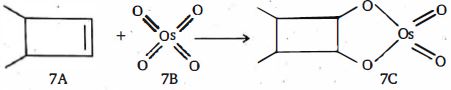
- 2$7B$ અને $7C$ માં ઓસ્મિયમ ની ઓક્સિડેશન અવસ્થા અનુક્રમે શું હશે ?View Solution
- 3${N_2}{H_4} + Cl{O_3}^ - \, \to \,NO\, + \,C{l^ - }$ (બેઝિક માધ્યમ) રેડોક્ષ પ્રક્રિયામાં ઓક્સિડેશન આંકના તફાવતને આધારે રિડક્શન અર્ધપ્રક્રિયામાં કેટલા ઇલેક્ટ્રોન ઉમેરાય ?View Solution
- 4વિધાન $I$ : ઘન $S_8$ બેઝિક પરિસ્થિતિમાં વિષમીકરણ પામી $\mathrm{S}^{2-}$ અને $\mathrm{S}_2 \mathrm{O}_3^{2-}$ બનાવે છે.View Solution
વિધાન $II$: $\mathrm{ClO}_4^{-}$એ એસીડીક પરિસ્થિતિમાં વિષમીકરણ પામે છે. તો યોગ્ય વિકલ્પ પસંદ કરો.
- 5બ્રાઉન રંગનો રિંગ સંકીર્ણ સંયોજન $\left[ {Fe{{\left( {{H_2}O} \right)}_5}\left( {NO} \right)} \right]S{O_4}$ છે, જેમાં આયર્નનો ઓ.આંક કેટલો હશે ?View Solution
- 6View Solutionનીચેનામાંથી ઓક્સિડાઇઝીંગ પદાર્થ ક્યો છે?
- 7આપેલ પ્રક્રિયા માટે $A, B, C, D$ પસંદ કરો. ${H_3}P{O_2} + AgN{O_2} \longrightarrow Ag \downarrow + {H_3}P{O_4} + NO$View Solution
- 8View Solutionક્રોમેટ અને ડાયક્રોમેટ આયનમાં ક્રોમિયમ અને ઓક્સિજન પરમાણુઓ વચ્ચેના કુલ બંધની સંખ્યા જણાવો.
- 9આપેલ પ્રક્રિયા માટે $A, B, C, D$ પસંદ કરો. $Mg\left( s \right) + 2HCl \longrightarrow MgC{l_2} + {H_2}$View Solution
- 10View Solutionગરમ સાંદ્ર સલ્ફ્યુરિક એસિડ એ સાધારણ પ્રબળ ઓક્સિડેશનકર્તા છે. નીચેનામાંથી કઈ પ્રક્રિયાઓ ઓક્સિડેશન વર્તણૂક બતાવતા નથી?
