$\underset{A}{\mathop{PhF}}\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\underset{B}{\mathop{PhCl}}\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\underset{C}{\mathop{PhBr}}\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\underset{D}{\mathop{PhI}}\,$
$(a)$ That the steric factor is not the sole determinant is, however, seen in the figures for the nitration of the halobenzenes, which are $o-/p-$ directing but on which overall attack is slightly slower than on benzene.
Despite the increase in size of the substituent $Y$ from $F \to I$, the proportion of $o-$ isomer increases. An increasing steric effect will, as with the alkyl benzenes, be operating to inhibit $o-$ attack, but this must here be outweighed by the electron-withdrawing inductive/field effect exerted by the halogen atom $(Y)$. This effect will tend to decrease with distance from $Y$, being exerted somewhat less strongly on the distant $p-$ position compared with the adjacent $o-$ position. Electronegative $F,$ and relatively little $o-$ attack thus takes place on $C_6 H_5F$, despite the small size of $F.$ The electron- withdrawing effect of the halogen $(Y)$ decreases considerably from $F$ to $I$ (the biggest change being between $F$ and $Cl$), resulting in increasing attack at the $o-$ position despite the increasing bulk of $Y.$
$Increase\,\,in\,\,size\,\,of\,\,Y$ $\begin{gathered}
\downarrow \hfill \\
\downarrow \hfill \\
\downarrow \hfill \\
\downarrow \hfill \\
\end{gathered} $ $\begin{array}{*{20}{c}}
Y&{\% \,o\, - }&{\% \,p\, - } \\
F&{12}&{88} \\
{Cl}&{30}&{69} \\
{Br}&{37}&{62} \\
I&{38}&{60}
\end{array}$
Download our appand get started for free
Similar Questions
- 1View Solutionકઈ ખોટી ઈલેકટ્રોમેરિક અસર છે ?
- 2પદાર્થની વધતી એસિડિકતાનો ક્રમ કયો છે ?View Solution
(1) $CH_2=CH_2$
(2) $[Figure]$
(3) $H-C \equiv C-H$
(4) $NH_3$
- 3View Solutionનીચેનામાંથી કયો પ્રબળ બેઈઝ છે ?
- 4View Solution....... માં બધી જ કાર્બન -કાર્બન બંધ લંબાઈઓ સમાન હોય છે.
- 5View Solutionસૂર્યપ્રકાશમાં એલિવેટેડ તાપમાને ટોલ્યુઇન અને ક્લોરિનની પ્રક્રિયામાં રચાયેલી મધ્યવર્તી શું રજૂ કરે છે?
- 6View Solutionએન્થ્રેસીન માટે કેટલા સંસ્પંદન બંધારણ છે?
- 7View Solutionનીચેનામાંથી કયો પદાર્થ હાઈડ્રોકઝીલીક પ્રોટોનથી સૌથી એસિડિક છે ?
- 8View Solutionનીચેનામાંથી કયા સમઘટકીય બંધારણમાં સૌથી ઓછી ઉર્જા છે?
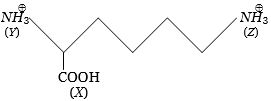
- 9નીચે આપેલા સંયોજનમાં$(X)$, $ (Y)$ અને $(Z)$ ની એસિડિટીનો યોગ્ય ક્રમ છે?View Solution
- 10View Solutionકયા બંધારણ સૌથી સ્થાયી છે ?
