\(l=0\) to \(( n +1)\)
\(n =1 \quad n =2\)
\(l=0,1,2 \quad l=0,1,2,3\)
\((n+l) \Rightarrow \frac{1 s}{1} \frac{1 p}{2} \frac{1 d}{3} \quad \frac{2 s}{2} \frac{2 p}{3} \frac{2 d}{4} \frac{2 f}{5}\)
\(n=3\)
\(l=0,1,2,3,4\)
\(\frac{3 s }{3} \frac{3 p }{4} \frac{3 d }{5} \frac{3 f }{6} \frac{3 g }{7}\)
Now, in order to write electronic configuration, we need to apply \(( n +l)\) rule
Energy order : \(1 s <1 p <2 s <1 d <2 p <3 s <2 d\)
Option 1) \(13: 1 s^{2} 1 p^{6} 2 s^{2} 1 d^{3}\) is not half filled
Option 2) \(9: \quad 1 s^{2} 1 p^{6} 2 s^{1}\) is the first alkali metal because after losing one electron, it will achieve first noble gas configuration
Option \(3)\) \(8: \quad 1 s^{2} 1 p^{6}\) is the first noble gas because after \(1 p ^{6} e ^{-}\) will
Option \(4)\) \(6: \quad 1 s ^{2} 1 p ^{4}\) has \(1 p\) valence subshell.
Download our appand get started for free
Similar Questions
- 1View Solutionનીચેનામાંથી કયા આયનોનો જોડ, સમઈલેક્ટ્રોનીક આયનોનો સમૂહ દર્શાવે છે?
- 2View Solutionકેથોડ કિરણોના સંદર્ભમાં નીચેના પૈકી ક્યુ વિધાન ખોટુ છે?
- 3કાલ્પનિક પરિસ્થિતિને ધ્યાનમાં લો જ્યાં ગૌણ ક્વોન્ટમ આંક $l$$0$ , $1,2, \ldots \ldots n+1,$ ની કિંમતો લે છે જ્યાં $n$ એ મુખ્ય ક્વોન્ટમ આંક છે ત્યારે અણુ સંખ્યા સાથેનું તત્વView Solution
- 4નીચેનામાંથી કઈ કક્ષક કે જેમાં ઇલેક્ટ્રોનનો સ્પિન ક્વોન્ટમ આંક $ + \frac{1}{2}$ તથા ચુંબકીય ક્વોન્ટમ આંક $ -\, 1$ શક્ય નથી.View Solution
- 5View Solutionનીચેનામાંથી શામાં આઉફબાઉ અને હૂન્ડના નિયમનુ પાલન થતુ નથી ?
- 6એક કાલ્પનિક વિદ્યુતયુંબકીય તરંગ નીચે દર્શાવેલ છે.View Solution
(Image)
તરંગની આવૃત્તિ $\mathrm{x} \times 10^{19} \mathrm{~Hz}$છે. $x=$ ............... (નજીક નો પૂર્ણાક)
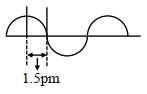
- 7હાઈડ્રોજન વર્ણપટમાં કઈ સંક્રાંતિએ $He { }^{+}$વર્ણપટમાં બામર પ્રકારની સંક્રાંતિમાં $n =4$ માંથી $n =2$ જેવી જ સમાન તરંગલંબાઈ ધરાવતી હશે ?View Solution
- 8View Solutionકયા સંક્રમણમાં એક ક્વોન્ટમ ઊર્જાનું ઉત્સર્જન?
- 9પરમાણુના $M$ કોષમાં $13$ ઇલેકટ્રોન હોય અને $N$ કોષમાં $1$ ઇલેકટ્રોન હોય તો તે પરમાણુમાં અયુગ્મિત ઇલેકટ્રોનની સંખ્યા ......View Solution
- 10View Solutionસોડિયમનાં સંયોજકતાં ઇલેકટ્રોન માટે કયો સેટ સાચો છે?
