$Ca{C_2} + 2{H_2}O \to Ca{(OH)_2} + {C_2}{H_2}$
${C_2}{H_2} + {H_2} \to {C_2}{H_4}$
$n({C_2}{H_4}) \to {( - C{H_2} - C{H_2} - )_n}$
$64.1\, kg$ $Ca{C_2}$માંથી મેળવેલ પોલિઇથિલિનનો જથ્થો ......$kg$ છે.
(d) $\mathop {Ca{C_2}}\limits_{{\rm{64}}\,g} + 2{H_2}O \to Ca{(OH)_2} + {C_2}{H_2}$
${C_2}{H_2} + {H_2} \to \mathop {{C_2}{H_4}}\limits_{{\rm{28}}\,g} $
$64\,g$ of $Ca{C_2}$ gives $28\,g$ of ethylene
$\therefore $ $64\,kg$ of $Ca{C_2}$ will give $28\,kg$ of polyethylene
Download our appand get started for free
Similar Questions
- 1View Solutionનીચેના ત્રણ ઉત્પાદનો આપવા માટે ટ્રાઇન ને એસીટીકએસિડમાં ઝીંક પછી ઓઝોન દ્વારા સારવાર આપવામાં આવે છે. તો ટ્રાઈન ની રચના શું છે?
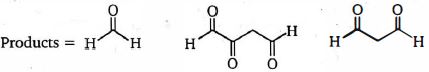
- 2સંયોજન $(iii)$ ને બાકીના સંયોજનોથી અલગ ઓળખવા માટે નીચેના પૈકી સૌથી યોગ્ય પ્રક્રિયક ક્યો છે ?View Solution
$(i)$ $C{H_3} - C \equiv C - C{H_3}$
$(ii)$ $C{H_3} - C{H_2} - C{H_2} - C{H_3}$
$(iii)$ $C{H_3} - C{H_2} - C \equiv CH$
$(iv)$ $C{H_3} - CH = C{H_2}$
- 3$CH_2 = CH_2$ ના ઓઝોનોલિસિસ દરમિયાન જો $LiAlH_4$ વડે રિડક્શન કરવામાં આવે તો મળતી નીપજ .............. થશે.View Solution
- 4નીચેની પ્રક્રિયા કઈ પ્રક્રિયા છે?View Solution
$C{H_2} = CH - C{H_3} + HBr \to C{H_3}CHBr - C{H_3}$
- 5નીચેની પ્રક્રિયાની મુખ્ય નીપજ કઈ છે ?View Solution
$\begin{array}{*{20}{c}}
{C{H_3}\,\,\,\,\,\,\,} \\
{|\,\,\,\,\,\,\,\,\,\,\,\,\,} \\
{{H_3}C - C - CH = C{H_2}} \\
{|\,\,\,\,\,\,\,\,\,\,\,\,\,} \\
{C{H_3}\,\,\,\,\,\,\,\,\,}
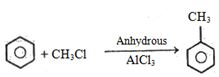
\end{array}$ $\xrightarrow{{{H_2}O/{H^ \oplus }}}{\mkern 1mu} \mathop A\limits_{Major\,product} \, + \,\mathop B\limits_{Minor\,product} $ - 6View Solutionફ્રિડલ-ક્રાફટ્સ પ્રક્રિયામાં ટોલ્યુઇન...... વડે બનાવી શકાય.
- 7View Solutionનીચેના સંયોજન માટે ઈલેકટ્રોનુરાગી વિસ્થાપનનો ચડતો ક્રમ
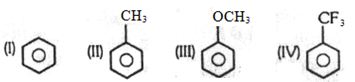
- 8હવાની હાજરીમાં $V_2O_5$ દ્વારા બેન્ઝિનનું ઑક્સિડેશન ઉત્પન્ન થાય છે ઊંચા તાપમાને શું મળે છે ?View Solution
- 9View Solutionપ્રક્રિયા કઇ છે ?
- 10નીચેનામાંથી ક્યા સંયોજનનું ઓઝોનોલિસિસ કરવાથી $5-$ કિટો $-2-$ મિથાઇલ હેકઝેનાલ મળે છે ?View Solution
