A hydrogen atom consists of an electron and a proton.
\(\therefore\) Charge on one hydrogen atom
\(=q_{e}+q_{p}=-e+(e+\Delta e)=\Delta e\)
Since a hydrogen atom carry a net charge \(\Delta e\)
\(\therefore \quad\) Electrostatic force,
\(F_{e} \frac{1}{4 \pi \varepsilon_{o}} \frac{(\Delta e)^{2}}{d^{2}}.........(i)\)
will act between two hydrogen atoms.
The gravitational force between two hydrogen atoms is given as
\(F_{g}=\frac{G m_{h} m_{h}}{d^{2}}.........(ii)\)
since, the net force on the system is zero, \(F_{e}=F_{g}\) Using eqns. \((i)\) and \((ii)\), we get
\({\frac{(\Delta e)^{2}}{4 \pi \varepsilon_{o} d^{2}}=\frac{G m_{h}^{2}}{d^{2}}}\)
\({(\Delta e)^{2}=4 \pi \varepsilon_{o} G m_{h}^{2}}\)
\({=6.67 \times 10^{-11} \times\left(1.67 \times 10^{-27}\right)^{2} /\left(9 \times 10^{9}\right)}\)
\({\Delta e=10^{-37} \,\mathrm{C}}\)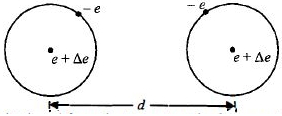
Download our appand get started for free
Similar Questions
- 1બે ઈલેક્ટ્રોનને $'2d'$ અંતરે જડિત રાખવામાં આવ્યા છે. એક ત્રીજા વિદ્યુતભાર પ્રોટોન કે જે મધ્યબિંદુએ રાખી તેને $x (x < < d)$ જેટલા ખૂબ નાના અંતરે બે જડીત વિદ્યુતભારોને જોડતી રેખાને લંબ ખસેડવામાં આવે છે. પ્રોટોન ......... કોણીય આવૃત્તિ સાથે સરળ આવર્ત ગતિ કરે છે. $(m \, =$ વિધુતભારિત કણનું દળ$)$View Solution
- 2$R$ ત્રિજયાના ગોળા પર $2Q$ જેટલો કુલ વિદ્યુતભાર છે જેની વિદ્યુતભાર ઘનતા $\rho(r) = kr$ જ્યાં $r$ એ કેન્દ્રથી અંતર છે. બે વિદ્યુતભાર $A$અને $B$ જેનો વિદ્યુતભાર $-Q$ છે તેને ગોળાના વ્યાસ પર કેન્દ્ર થી સમાન અંતર પર છે. જો $A$ અને $B$ પર કોઈ બળ લાગતું ના હોય તો.....View Solution
- 3$4\,\mu\,C$ વિદ્યુતભારને બે ભાગ માં વહેંચવામાં આવે છે. જુદા પાડેલા આ બન્ને વિદ્યુતભારો વચ્ચેનું અંતર અચળ છે. જુદા પાડેલ આ વિદ્યુતભારો વચ્ચે લાગતું બળ મહત્તમ થાય તે માટે વિદ્યુતભારોનું મૂલ્ય $..........$ થશે.View Solution
- 4દરેક $m$ જેટલું દળ અને $q$ જેટલો વિદ્યુતભાર ધરાવતા બે એકસમાન ટેનિસ બોલને $l$ લંબાઈની દોરી વડે જડિત બિંદુથી લટકવવામાં આવેલ છે. જ્યારે શિરોલંબ સાથે દરેક દોરી નાનો કોણ $\theta$ રચતી હોય તો ત્યારે સંતુલન સ્થિતિમાં અંતર .......... હશે?View Solution
- 5View Solutionઆકૃતિમાં દર્શાવ્યા અનુસાર એક ડાયપોલને વિદ્યુતક્ષેત્રમાં મૂકવામાં આવે છે. તે કઈ દિશામાં ગતિ કરશે?
- 6સમબાજુ ત્રિકોણના $A$ બિંદુ પર રહેલાં વિદ્યુતભાર પર $BC$ ને લંબ દિશામાં કેટલું બળ લાગે?View Solution
- 7ત્રણ ધન $q$ મૂલ્યના વિજભાર ત્રિકોણના શિરોબિંદુ પર પડેલા છે.તેની પરિણામી બળ રેખા કેવી દેખાય?View Solution
- 8View Solutionઆકૃતિ વિદ્યુતક્ષેત્ર સાથે (સંલગ્ન) કેટલીક વિદ્યુત રેખાઓ દર્શાવે છે. તો......
- 9જ્યારે પોલીથીનના એક ટુકડાને ઊન સાથે ઘસવામાં આવે તો પોલીથીન પર $-2 -\times 10^{-7}\ C$ જેટલો વિદ્યુતભાર ઉત્પન્ન થાય છે. દળનો કેટલો જથ્થો પોલીથીન તરફ વહન પામતો હશે?View Solution
- 10એક લાંબા નળાકારીય કદ ધનતા $\rho$ ધરાવતું નિયમિત વિદ્યુતભાર વિતરણ ધરાવે છે. નળાકારીય કદની ત્રિજ્યા $R$ છે. એક $q$ વિદ્યુતભારીત કણ તેની આસપાસ વર્તુળાકાર પથ પર ભ્રમણ કરે છે. વિદ્યુતભારની ગતિઉર્જા ......થશે.View Solution
