(આપેલ: $ E^oCr^{+3}| Cr = -0.75 \,V$ $E^o Fe^{+2} | Fe = - 0.45\, V)$
અર્ધકોષીય પ્રક્રિયા
એનોડ પર,\( \Rightarrow \,\,[Cr \to C{r^{ + 3}} + 3{e^ - }]\,\, \times \,\,2\)
કેથોડ પર \( \Rightarrow \,\,[F{e^{ + 2}} + 2{e^ - } \to Fe]\,\, \times \,\,3\)
સંપૂર્ણ પ્રક્રિયા \({2Cr + 3F{e^{ + 2}} \to 2C{r^{ + 3}} + 3Fe}\)
\(E_{cell}^ o= \) ઓક્સિડેશન પોટેન્શિયલ \(+\) રિડકશન પોટેન્શિયલ
\( = 0.75+( -\,0.45)=0.30\)
\({E_{cell}}\, =E_{cell}^o - \frac{{0.059}}{n}\,\log \) [પ્રક્રિયા]/[પ્રક્રિયા]
\( = 0.30- \frac{{0.059}}{6}\,\log \frac{{{{[C{r^{ + 3}}]}^2}}}{{{{[F{e^{ + 2}}]}^3}}}\)
\( = \,0.30\, - \frac{{0.059}}{6}\,\log \,\frac{{{{[0.1]}^2}}}{{{{[0.01]}^3}}}\)
\(\, = \,0.30\, - \,\frac{{0.24}}{6}\,= \,0.26\,Volt.\)
Download our appand get started for free
Similar Questions
- 1View Solutionહાઈડ્રોજન અર્ધકોષનો રિડકશન પોટેન્શિયલ ઋણ ત્યારે હોય છે, જ્યારે .........
- 2કોષનો $emf \,0.83 \,V$ છે. $Tl_{(s)} | Tl^{+}_{(aq)}\, (0.0001\,M) | | Cu^{2+}_{(aq)}\, (0.01\,M) | Cu_{(s)}$ આ કોષનો $emf $ કોના દ્વારા વધે છે?View Solution
- 3$298\, K$ તાપમાને $Zn | ZnSO_{4(0.01\, M)} | | CuSO_{4(1 M)} | Cu$ ડેનિયલ કોષનો પોટૅન્શિયલ $E_1$ છે. $ZnSO_4$ અને $CuSO_4$ ના દ્રાવણની સાંદ્રતા અનુક્રમે $1\, M$ અને $0.01 $ લેવામાં આવે, તો કોષનો પોટૅન્શિયલ બદલાઈને મળે છે, તો $E_1$ અને $E_2$ વચ્ચેનો સંબંધ જણાવો.View Solution
- 4વિધુતરસાયણિક કોષના $emf$ માટે નીચેના સંબંધ વિચારો.View Solution
$(i)$ કોષનો $EMF$ $=$ (એનોડનો ઓક્સિડેશન પોટેન્શિયલ) $-$ (કેથોડનો રીડકશન પોટેન્શિયલ)
$(ii)$ કોષનો $EMF$ $=$ (એનોડનો ઓક્સિડેશન પોટેન્શિયલ) $+$ (કેથોડનો રીડકશન પોટેન્શિયલ)
$(iii)$ કોષનો $EMF$ $=$ (એનોડનો રીડકશન પોટેન્શિયલ) $+$ (કેથોડનો રીડકશન પોટેન્શિયલ)
$(iv)$ કોષનો $EMF$ $=$ (એનોડનો ઓક્સિડેશન પોટેન્શિયલ) $-$ (કેથોડનો ઓક્સિડેશન પોટેન્શિયલ)
નીચેના પૈકી ક્યા સંબંધો સાચા છે ?
- 5પીગાળેલ $CaCl _{2}$ (પરમાણ્વીય દળ $,$ $\left. Ca =40\, g\, mol ^{-1}\right)$ માંથી $20\, g$ કેલ્શિયમનું ઉત્પાદન કરવા માટે કેટલા ફેરાડે $(F)$ની સંખ્યા જરૂરી છે?View Solution
- 6કોષ પ્રક્રિયા $2 \mathrm{Fe}^{3+}(\mathrm{aq})+2 \mathrm{I^-}(\mathrm{aq}) \rightarrow 2 \mathrm{Fe}^{2+}(\mathrm{aq})+\mathrm{I}_{2}(\mathrm{aq})$ માટે $298$ $K$ તાપમાને $\mathrm{E}_{\text {call }}^{\mathrm{e}}=0.24 \mathrm{V}$ છે. કોષ પ્રક્રિયા માટે પ્રમાણિત ગિબ્સ ઊર્જા $\left( {{\Delta _r}{{\rm{G}}^ \ominus }} \right)$ શું થશે? (ફેરાડે અચળાંક ${\rm{F}} = 96500\;{\rm{C}}\,{\rm{mo}}{{\rm{l}}^{ - 1}}$ આપેલ છે)View Solution
- 7View Solutionનીચેનામાંથી કઈ ધાતુ તેના ક્ષારના જલીય દ્રાવણના વિદ્યુતવિભાજય પર મેળવી શકાતી નથી.
- 8પિગલીત કેલ્શિયમ હાઈડ્રાઈડ $(CaH_2)$ ના વિદ્યુત વિભાજન દરમિયાન હાઈડ્રોજન ક્યાં ઉત્પન્ન થાય?View Solution
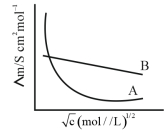
- 9સાંદ્રતા પર બે વિદ્યુતવિભાજ્યોની મોલર વાહકતા નો આધાર $(dependence)$ નીચે આકૃતિમાં દર્શાવેલ છે.$\Lambda \stackrel{\circ}{m}$ એ સિમિત મોલર વાહક્તા છે.View Solution
નીચે આપેલા માંથી ખોટા વિધાન(નો)ની સંખ્યા $..........$ છે.
$(A)$ $\Lambda \stackrel{0}{ m }$ for electrolyte $A$ is obtained by extrapolation
$(B)$ વિદ્યુતવિભાજ્ય $B$ માટે $\Lambda m$ વિરૂદ્ધ $\sqrt{c}$ આલેખ સીધી રેખા મળે છે અને સાથે આંતરછેદ એ $\Lambda \stackrel{0}{ m }$ ને બરાબર (સમાન) છે.
$(C)$ અનંત મંદન પર વિદ્યુતવિભાજ્ય $B$ માટે વિયોજન અંશ નું મૂલ્ય શૂન્ય પ્રસ્થાપિત કરે છે.
$(D)$ વિદ્યુતવિભાજ્ય $A$ અથવા $B$ માટે $\Lambda \stackrel{0}{ m }$ વ્યક્તિગત આયનો માટે $\lambda^{\circ}$ નો ઉપયોગ કરીને ગણતરી કરી શકાય છે ?
- 10$NaCl$ કોષમાંથી $16\,A$ પ્રવાહ $10\, min$ માટે પસાર કરતાં કેટલા ............... $\mathrm{gm}$ ધાત્વિક $Na$ એ ઋણ ઇલેક્ટ્રોડ પર જમા થશે?View Solution
