કયા ઇલેક્ટ્રોનનું સ્તર હાઇડ્રોજન અણુને ફોટોન શોષી લેવાની મંજૂરી આપશે પરંતુ ફોટોનને બહાર કાઢશે નહીં ?
IIT 1984, Medium
d
\(1s\) electronic level would allow the hydrogen atom to absorb a photon but not to emit it. On absorption of radiation, electron will be transferred from is level to higher level such as \(2 s\) or \(3 s\). But to emit a photon, the electron should go to the lower energy level from higher energy level. Since is is the lowest energy level, the electron cannot go to a lower level. Hence, emission is not possible.
\(1s\) electronic level would allow the hydrogen atom to absorb a photon but not to emit it. On absorption of radiation, electron will be transferred from is level to higher level such as \(2 s\) or \(3 s\). But to emit a photon, the electron should go to the lower energy level from higher energy level. Since is is the lowest energy level, the electron cannot go to a lower level. Hence, emission is not possible.
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1$10^8 \,cm/s$ જેટલો વેગ ધરાવતાં ઈલેકટ્રોન સાથે સંકળાયેલ તરંગ લંબાઈ ........ $\mathop {\rm{A}}\limits^{\rm{o}} $ છે.View Solution
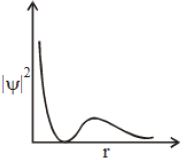
- 2${\left| \psi \right|^2}$ અને $r$(radial distance) વચ્ચેનો આલેખ નીચે દર્શાવ્યો છે . તે .. . રજૂ કરે છે .View Solution
- 3View Solutionવિધાન : પ્રોટોન અને ન્યુટ્રોનનો સરવાળો આઇસોબારમાં હંમેશા અલગ હોય છે.
કારણ :આઇસોબાર્સ વિવિધ તત્વોના પરમાણુ છે જે એક સમાન દળ સંખ્યા ધરાવે છે પરંતુ વિવિધ અણુ સંખ્યા હોય છે. - 4$5800\,\mathop A\limits^o $ તરંગલંબાઇ ધરાવતા વિકિરણની આવૃતિ ... $s^{-1}$ છે.View Solution
- 5$H$ પરમાણુની ઊર્જા ભૂમિ. અવસ્થામાં $-13.6\, eV$, આપેલ છે. જેથી બીજી ઉત્તેજીત અવસ્થાની ઊર્જા .......... $\mathrm{eV}$ થશે.View Solution
- 6View Solutionનીચેના માંથી સાયક્લોટ્રોન કોને પ્રવેગિત કરી શકતું નથી ?
- 7આપેલા $H$ પરમાણુમાં $n = 1$ થી $n = 2$ ઊર્જાનું સંક્રમણ થતાં રીડબર્ગ સૂચવ્યું તે $10.2\,eV$ નળે છે ત્યારે એજ સ્થિતિમાં $Be^{3+}$ માં એજ સંક્રમણ માટેની ઊર્જા ................. $\mathrm{eV}$ થશે.View Solution
- 8View Solutionકયા ઇલેક્ટ્રોનિક સ્તરથી હાઇડ્રોજન અણુ ફોટોનને શોષી લેવાની મંજૂરી આપશે પરંતુ ફોટોનને બહાર કાઢવા માટે નહીં
- 9$Cl_2$ માં રહેલા એક મોલ $Cl-Cl$ બંધ તોડવા જરૂરી ઊર્જા $242\, kj \, mol^{-1}$ છે. તો એક $Cl-Cl$ બંધ તોડવા જરૂરી પ્રકાશની મહતમ તરંગલંબાઈ .......... $\mathrm{nm}$ થશે.View Solution
$(C= 3 \times 10^8 \,ms^{-1},N_A = 6.02 \times 10^{23} mol^{-1}).$
- 10View Solutionનીચે આપેલા સમૂહ માંથી સમ-ઇલેક્ટ્રોનીય સમૂહ પસંદ કરો ?
