$\left[{Ni}({CN})_{6}\right]^{2-}$ની રચના કરવા માટે મજબૂત ઓક્સિડાઇઝિંગ એજન્ટની હાજરીમાં ${NiCl}_{2}$નું જલીય દ્રાવણ વધારે સોડિયમ સાયનાઇડ સાથે ગરમ કરવામાં આવ્યું હતું. કેન્દ્રીય ધાતુ પર અયુગ્મિત ઇલેક્ટ્રોનની સંખ્યામાં કુલ ફેરફાર $.....$ છે.
JEE MAIN 2021, Diffcult
c
$\left[{Ni}({CN})_{6}\right]^{2-}$
$\left[{Ni}({CN})_{6}\right]^{2-}$
${Ni}^{+4} \rightarrow {d}^{6}$ strong field ligand
$[image(1)]$
Pairing will be there zero unpaired electron
${NiCl}_{2} \rightarrow {Ni}^{2+} \rightarrow {d}^{8}$
$[image(2)]$
Change $=2$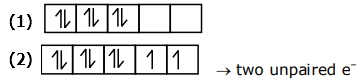
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1View Solutionકયું સંયોજન ચતુષ્ફલકીય આકાર ધરાવે છે?
- 2$[Co{(N{H_3})_5}S{O_4}]\,Br$ નું $X = 0.02$ મોલ નું મિશ્રણ અને $[Co{(N{H_3})_5}Br]S{O_4}$ નું $0.02$ મોલ મિશ્રણ દ્રાવણ $2$ લિટર દ્રાવણ $1$ લિટર મિશ્રણવધુ $X$ $+$ $AgN{O_3} \to Y$. માં તૈયાર કરવામાં આવ્યું હતું.$1$ લિટર મિશ્રણ $X$ + વધુ માત્રા $BaC{l_2} \to Z$ તો $Y$ અને $Z$ ના મોલ ની સંખ્યા કેટલી હશે ?View Solution
- 3$Fe(CO)_5$ ના $IUPAC$ નામ....View Solution
- 4View Solutionનીચે પૈકી કયું વિધાન ખોટું છે?
- 5View Solutionફેરિક ક્ષારમાં પોટેશિયમ ફેરોસાયલાઇડ ઉમેરવાથી પ્રુસીયન બ્લૂ રંગ મળે છે, જે મુખ્યત્વે ......... બનવાને લીધે છે.
- 6$[CrCl_2(H_2O)_4]NO_3$ સંકીર્ણનું $IUPAC$ નામ.....View Solution
- 7$Mn^{4+}$ આયનની અનુચુંબકીય ચાકમાત્રા આશરે ...... $B.M.$View Solution
- 8View Solutionનીચેનામાંથી ફેસિયલ અને મેરિડિયોન પ્રકારની સમઘટતા કોણ ધરાવી શકશે ?
- 9$[CO(NH_3)_5Br]SO_4$ અને $[CO(NH_3)_5SO_4]Br$ ..... સમઘટક છે.View Solution
- 10View Solutionનીચેના પૈકી કયુ hemoleptic સંકીર્ણનું ઉદાહરણ છે?
