મધ્યસ્થઅણુ શૂન્ય પર નીચેની સંખ્યામાંથી અયુગ્મ જોડી કઈ છે
$XeO _{3}, XeO _{2} F _{2}, XeO _{4}, XeO _{3} F _{2}, Ba _{2} XeF _{4}$
AIIMS 2019, Medium
a
The geometries of the given compounds are shown below.
The geometries of the given compounds are shown below.
Among the above shown compounds $XeO _{4},$ and $XeO _{3} F _{2}$ do not have lone pairs of electrons.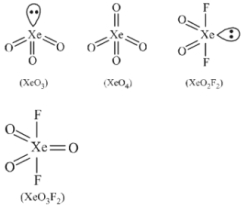
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1$\mathrm{CO}$ અને $\mathrm{NO}^{+}$ ના બંધક્રમાંકનો સરવાળો ___________છે.View Solution
- 2નીચે આપેલામાંથી અધ્રુવીય આણુઓની સંખ્યા_______________ છે.View Solution
$\mathrm{HF}, \mathrm{H}_2 \mathrm{O}, \mathrm{SO}_2, \mathrm{H}_2, \mathrm{CO}_2, \mathrm{CH}_4, \mathrm{NH}_3, \mathrm{HCl}, \mathrm{CHCl}_3, \mathrm{BF}_3$
- 3$Xe{F_2}$ માં $Xe$ ના સંકરણનો પ્રકાર ક્યો છે?View Solution
- 4$N_2$ ના દરેક બાહ્ય કૌસ દ્વારા વહેચાયેલ ઈલેક્ટ્રોનની સંખ્યા શું હશેView Solution
- 5$o, p$ અને $m-$ ડાયક્લોરોબેન્ઝિનનો દ્વિધ્રુવીય ચાકમાત્રા ક્યાં ક્રમમાં હશે?View Solution
- 6View Solutionનીચે પૈકી ઘટકોની કઇ જોડી સમાન આકાર ધરાવે છે?
- 7View Solutionકયો બંધ સૌથી વધુ બંધ-ઊર્જા ધરાવે છે?
- 8$BrF_{3}$ અણુમાં મધ્યવર્તી પરમાણુમાં અસંબંધકારક યુગ્મ(મો)ની સંખ્યા અને આકાર,...... .View Solution
- 9View Solutionમજબુત બંધ ક્યો છે
- 10View Solutionનીચેનામાંથી કઇ જોડમાં બે ઘટકો સમબંધારણીય નથી ?
