$N_2, O_2, O_2^-$ પૈકી બંધઉર્જાનો સાચો ક્રમ નીચેના દર્શાવેલી કઈ ગોઠવણીમાં છે ?
JEE MAIN 2014, Medium
c
The bond order of \(N_2,\) \(O_2,\) and \(O_2^-\) are respectively \(3,2\) and \(1.5\)
The bond order of \(N_2,\) \(O_2,\) and \(O_2^-\) are respectively \(3,2\) and \(1.5\)
Since higher bond order implies higher bond dissociation energy hence the correct orde will be
\(N_2 > O_2 > O_2^-\)
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1જો તત્વ $X$ ની અણુ સંખ્યા $7$ છે, તો ક્યાં તત્વ માટે સૌથી ઉતમ ઈલેક્ટ્રોન બિંદુ સંકેત છેView Solution
- 2View Solutionનીચે આપેલામાંથી અણુઓની સંખ્યા કે જે હાઈડ્રોજન બંધન પ્રદર્શિત (દર્શાવી) કરી શકે છે તે ............
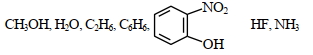
- 3$H_2O$ની ચોખ્ખી દ્વિધ્રુવીય ચાકમાત્રા છે જ્યારે $BeF_2$ શૂન્ય દ્વિધ્રુવીય ચાકમાત્રા છે કારણ કેView Solution
- 4$KO_2 , KAlO_2 , CaO_2$ અને $NO^+_2$ વચ્ચે, કયામાં અયુગ્મિત ઇલેક્ટ્રોન હાજર હોય છે?View Solution
- 5બરફમાં , $H-$બંધની લંબાઈView Solution
- 6View Solutionનીચેના માંથી શેનું ઉત્કલન બિંદુ ઊંચું છે?
- 7View Solutionસહસંયોજક સંયોજનો નીચેનામાંથી શેમાં દ્રાવ્ય હોય છે?
- 8ક્યા સંયોજનમાં સૌથી મોટો $H - M - H$ બંધકોણ છે?$( M = N , O , S , C )$View Solution
- 9$Si{(C{H_3})_4}$ નું બંધારણ અને સંકરણ કયુ છે?View Solution
- 10View Solutionઅણુઓ નીચેનામાંથી શું ધરાવતા હોય તો અનુચુંબકીય ગુણધર્મ દર્શાવે છે?
