નીચે આપેલા સંયુગ્મ બેઈઝો ની બેઝિક સામર્થ્ય ઘટતો ક્રમ શું થશે ?
${ }^{-} \mathrm{OH}, \mathrm{R} \overline{\mathrm{O}}, \mathrm{CH}_3 \mathrm{CO} \overline{\mathrm{O}}, \mathrm{C} \overline{1}$
JEE MAIN 2024, Medium
b
Strong acid have weak conjugate base Acidic strength :
Strong acid have weak conjugate base Acidic strength :
$\mathrm{H}-\mathrm{Cl}>\mathrm{CH}_3 \mathrm{COOH}>\mathrm{H}_2 \mathrm{O}>\mathrm{R}-\mathrm{OH}$
Conjugate base strength :
$\mathrm{Cl}^{-}<\mathrm{CH}_3 \mathrm{COO}^{-}<\overline{\mathrm{O}} \mathrm{H}<\mathrm{RO}^{-}$
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1View Solutionસ્થાયી કાર્બોકેટાયન જે ઉપરની પ્રક્રિયામાં બને છે તે શોધો.
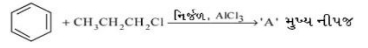
- 2ઇથાઈલ કાર્બોટાયનમાં ખાલી $p$ કક્ષા સાથે ઓવરલેપ માટે કેટલા કાર્બન-હાઇડ્રોજન બંધ ની કક્ષા હાજર છે?View Solution
- 3View Solutionઆપેલા પ્રમાણભૂત બંધારણણો સાચો સ્થાયિતા ક્રમ કયો છે ?
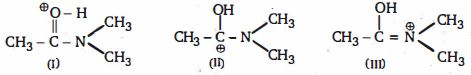
- 4વધતા દરના ક્રમમાં નીચેની પ્રતિક્રિયાઓ $A, B$ અને $C$ ને યોગ્ય ક્રમ આપો,View Solution
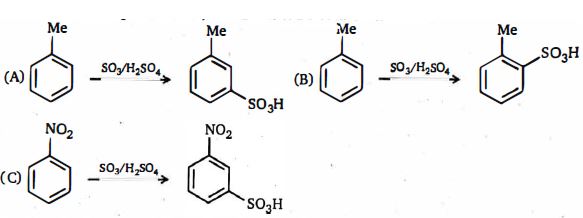
- 5$(I)$ $CH_3CH_2COOH $View Solution
$(II)$ $CH_2 = CH - COOH $ અને
$(III)$ $ HC \equiv C - COOH$
એસિડોની ગોઠવણી ઘટતી એસિડીકતા ક્રમમાં ગોઠવો.
- 6View Solutionસૌથી સ્થિર કાર્બએનાયન કયો છે ?
- 7View Solutionસૌથી પહેલા હાઇડ્રોજનની ઉષ્મામાં ઘટાડો કરવાના ક્રમમાં નીચે આપેલા આલકેન્સને ક્રમ આપો
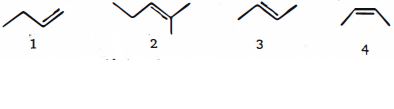
- 8ક્લોરોબેન્ઝિન માધ્યમમાં જુદા જુદા એમાઈનનો સાચો બેઝિકતા ક્રમ કયો છે ?View Solution
$(A) \,\,CH_3-CH_2-NH_2$
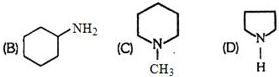
- 9View Solutionનીચેના પૈકી કયો અણુ સૌથી ઓછી સંસ્પંદનીય સ્થિરતા ધરાવે છે?
- 10એસિડિક પ્રબળતાના વધતા ક્રમમાં નીચેનાને ફરીથી ગોઠવો.View Solution
$(i)$ બેઞ્ઝોઈક એસિડ $(ii)\, p$ - મિથોક્સિબેઞ્ઝોઈક એસીડ $(iii)\, o$ - મિથોક્સિબેઞ્ઝોઈક એસિડ
