$1.\,\,(CH_3)_2 - \mathop C\limits^ + - CH_2 - CH_3$
$2.\,\,(CH_3)_3 - \mathop C\limits^ + $
$3.\,\,(CH_3)_2 - |\mathop C\limits^ + H|$
$4.\,\,CH_3 - \mathop C\limits^ + H_2$
$5.\,\,\mathop C\limits^ + H_3$
stability of carbocations follows following trendmethyl
$<1^{\circ}<2^{\circ}<3^{\circ}$
Given carbocations $(\mathrm{CH} _3) _3 \mathrm{C} \cdot \Rightarrow 3^{\circ}$ carbocation
$(\mathrm{CH} _3) _2 \mathrm{CH} \cdot=>2^{\circ}$ carbocation
$\mathrm{CH} _3 \mathrm{CH} _2 \cdot \Rightarrow 1^{\circ}$ carbocation
Therefore, increasing order of stability of carbocations $\mathrm{CH} _3 \mathrm{CH}_ 2 \cdot\left(1^{\circ}\right)<(\mathrm{CH}_ 3) _2 \mathrm{CH} \cdot\left(2^{\circ}\right)<(\mathrm{CH}_3)_ 3 \mathrm{C} \cdot\left(3^{\circ}\right)$
Download our appand get started for free
Similar Questions
- 1View Solutionએસિડ્સની કેટલીક જોડીઓ નીચે આપેઌ છે. તે જોડી પસંદ કરો જેમાં પ્રથમ એસિડ વધુ પ્રબળ હોય
- 2View Solutionનીચેનામાંથી કયો અણુ સૌથી વધુ ડાઈપોલ મોમેન્ટ ધરાવે છે ?
- 3નીચેના એનાયન ને ધ્યાન માં લો .View Solution
જ્યારે $sp^3$ કાર્બન સાથે જોડાયેલ હોય ત્યારે, ન્યુક્લિયોફિલિકવિસ્થાપન પ્રક્રિયામાં તેમની છોડવાની જૂથની ક્ષમતાનો કયા ક્રમમાં ઘટાડો થાય છે.
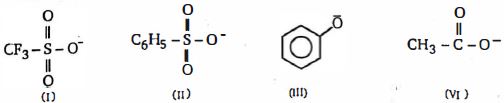
- 4View Solutionઇલેક્ટ્રોફાઇલ (ઇલેક્ટ્રોન અનુરાગી) ના સંદર્ભમાં સાચુ વિધાન પસંદ કરો.
- 5View Solutionનીચેના ઘટકોનો સ્થાયિતાનો સાચો ક્રમ જણાવો;
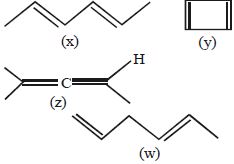
- 6કાર્બનની કઈ સ્પીસીઝ $e-$ નું ષષ્ટક ધરાવે છે અને ઈલેક્ટ્રોન અનુરાગી તરીકે વર્તે છે.View Solution
- 7View Solutionઆપેલ સંયોજનોમાં, એનોલ સામગ્રીનો યોગ્ય ક્રમ કયો છે
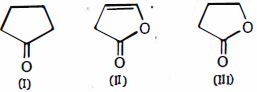
- 8$\overset{\oplus }{\mathop{C}}\,{{H}_{3}}\,$ નો આકાર કેવો છે?View Solution
- 9નિક્ટતમ પરમાણુ પર હાજર અબંધકારક ઈલેક્ટ્રોન યુગ્મ અને $\pi$ બંધ વચ્ચેની પારસ્પરિક ક્રિયા માટે જવાબદાર_____View Solution
- 10View Solutionનીચે આપેલા પૈકી ક્યું ખોટું વિધાન છે ?
