નીચે પૈકી કોણ મહત્તમ સ્થાયીતા ધરાવે છે?
AIIMS 2001, Medium
d
Stability of carbonium ion or carbocation depends on the adjacent alkyl groups. Alkyl groups exhibit electron releasing \(+ I\) effect and hyperconjugative effect which stabilizes the positive charge. \(CH _3^{+}\) methyl carbonium ion does not have both of these effects, therefore it is the least stable.
Stability of carbonium ion or carbocation depends on the adjacent alkyl groups. Alkyl groups exhibit electron releasing \(+ I\) effect and hyperconjugative effect which stabilizes the positive charge. \(CH _3^{+}\) methyl carbonium ion does not have both of these effects, therefore it is the least stable.
Therefore \(\begin{array}{*{20}{c}} {\,\,\,\,\,C{H_3}} \\ | \\ {\mathop {C{H_3} - C - C{H_3}}\limits_ + } \end{array}\) have maximum stability.
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1નીચેનામાંથી કયું સંયોજન ગરમ સાંદ્ર ${H_2}S{O_4}$માં પણ અદ્રાવ્ય છે?View Solution
- 2$1,2$-ડાયબ્રોમો ઇથેનની જસત ધાતુ સાથે ઊચા તાપમાનેે પ્રક્રિયા કરતાં મળતી નીપજ........ છે.View Solution
- 3પ્રક્રિયામાં $C{H_3} - C \equiv C - C{H_3}\xrightarrow[{(ii)\,{H_2}O/Zn}]{{(i)\,X}}\begin{array}{*{20}{c}}View Solution
{C{H_3} - C - C - C{H_3}} \\
{||\,\,\,\,\,\,\,\,\,\,||} \\
{O\,\,\,\,\,\,\,O}
\end{array};$ $X$ એ શું છે. - 4${C_6}{H_5} - C \equiv C - C{H_3}$ ${\mkern 1mu} \mathop {\xrightarrow{{HgS{O_4}}}}\limits_{{H_2}S{O_4}} {\mkern 1mu} {\mkern 1mu} A{\mkern 1mu} ..........$View Solution
- 5આલ્કાઇન $KMnO_4$ દ્રાવણનો રંગ દૂર કરતું નથી તથા ઍમોનિયમ સિલ્વર નાઇટ્રેટ સાથે અવક્ષેપ આપતું નથી આ હાઇડ્રોકાર્બન ....... છે.View Solution
- 6View Solutionનીચે પૈકી કયો બંધ ધરાવતો હાઇડ્રોકાર્બન સૌથી વધુ ક્રિયાશીલ છે?
- 7View Solutionનીચે આપેલા સંયોજનો પૈકી ક્યા એકનું સલ્ફોનેશન ખૂબ જ સરળતાથી થશે ?
- 8ઉપરની પ્રક્રિયામાં $X$ શોધો:View Solution
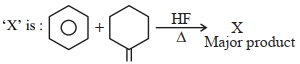
- 9નીચેની પ્રક્રિયાઓ ધ્યાનમાં લો , $A$ શું છે?View Solution
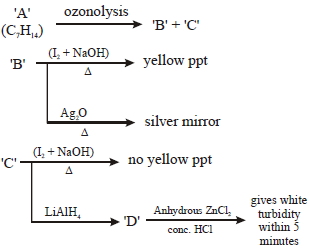
- 10$\begin{array}{*{20}{c}}View Solution
{2C{H_3} - C = C{H_2}} \\
{\,|} \\
{\,\,\,Ph}
\end{array}\xrightarrow{{H - A\,(acid)}}\mathop {(A)}\limits_{(major)} $નીપજ $(A)$ .........
