$(i)$ $\begin{array}{*{20}{c}}
{\begin{array}{*{20}{c}}
{\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,C{H_3}-CH_2} \\
{\,\,\,\,\,\,\,\,\,\,\,\,\,|}
\end{array}} \\
{C{H_3} - {C^ + }} \\
{\,\,\,\,\,\,\,\,\,\,\,\,\,\,|} \\
{\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,C{H_3}}
\end{array}$
$(ii)$ $\begin{array}{*{20}{c}}
{\begin{array}{*{20}{c}}
{\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,C{H_3}} \\
{\,\,\,\,\,\,\,\,\,\,\,\,\,|}
\end{array}} \\
{C{H_3} - {C^ + }} \\
{\,\,\,\,\,\,\,\,\,\,\,\,\,\,|} \\
{\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,{H}}
\end{array}$
$(iii)$ $\begin{array}{*{20}{c}}
{\begin{array}{*{20}{c}}
{\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,C{H_3}} \\
{\,\,\,\,\,\,\,\,\,\,\,\,\,|}
\end{array}} \\
{C{H_3} - {C^ + }} \\
{\,\,\,\,\,\,\,\,\,\,\,\,\,\,|} \\
{\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,C{H_3}}
\end{array}$
$(iv)$ $Ph - CH ^{+}-\underline{ CH }_{3}$
The stability of carbocation depends upon \(+ M\) effect, \(+ I\) effect and \(+ H\) effect.
Carbocation \((i)\) shows eight hyperconjugation structures.
Carbocation \((ii)\) shows six hyperconjugation structures.
Carbocation \((iii)\) shows nine hyperconjugation structures.
Carbocation \((iv)\) shows \(+M\) effect.
Therefore, the correct stability order is : \(iv > iii > i > ii\)
Download our appand get started for free
Similar Questions
- 1View Solutionનીચે આપેલા પૈકી એરોમેટિક ઈલેકટ્રોનઅનુરાગી વિસ્થાપન પ્રક્રિયામાં અક્રિયકારક સમૂહોની કુલ સંખ્યા__________ છે.
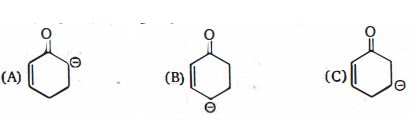
- 2View Solutionનીચેનામાંથી કયો સૌથી સ્થિર કાર્બોએનાયન છે ?
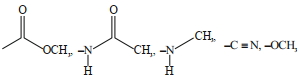
- 3આપેલા પરમાણુના નામાંકનમાં, $H$ અણું શામેલ છેView Solution
- 4$\begin{array}{*{20}{c}}View Solution
{O\,\,\,\,\,\,\,\,\,\,} \\
{||\,\,\,\,\,\,\,\,\,\,} \\
{C{H_3} - C\mathop {-} \limits_a O \mathop {-} \limits_b C{H_3}}
\end{array}$$a$ અને $b$ વચ્ચે ની બંધ લંબાઈ નો સાચો ક્રમ કયો છે ?
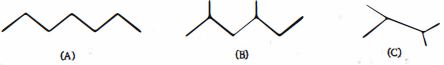
- 5દહન ઉષ્માના ક્રમમાં નીચેના પદાર્થોને ક્રમાંકિત કરો ( મોટાભાગના ઉષ્માશોષક $\to$ ઓછામાં ઓછા ઉષ્માશોષક)View Solution
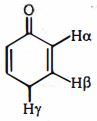
- 6View Solutionક્રિયાશીલ સમૂહ કે જે ઋણ સંસ્પંદન અસર દર્શાવે છે તે_________________.
- 7View Solutionબેઝિક ક્ષમતાનો સાચો ઘટતો ક્રમ કયો છે ?
- 8View Solutionનીચે આપેલા પૈકી ક્યું ખોટું વિધાન છે ?
- 9નીચે આપેલા સંયોજનોમાં $pK_b$ નો વધતો ક્રમ શું હશે?View Solution
- 10View Solutionઉપરોક્ત સંયોજનોની બેઝિક પ્રબળતાની તુલના કરો